

Design
Intro
We aim to create a new variation of targeting drug aimed are treating Breast Cancer. Our new drug delivery system is made up of three parts — Aptamer, Nanoparticle and the delivered drug.

Moreover, to make our system more specific and more stable, we plan to make some modifications on both aptamers and nanoparticles. For specificity, we will modify our aptamer to make it pH-sensitive. And for stability, we will attach chitosan and PEG to the nanoparticle, and PEG to the aptamer.
To see more on our Experimental Design, please go to our Experimental Design Page.
Aptamer
Aptamer Info
Aptamers are artificial single-stranded RNA or DNA oligonucleotides that can fold into 3D structures by which they can selectively and exclusively bind with specific biomarkers. In this way, aptamers function similar to antibodies, providing specificity to the targeted drug. A type of cancer cell normally has its own biomarker, resembling an identity card—due to the over-expression of various oncogenes, biomarkers have been associated with the development and progression of tumors. The way targeted drugs kill cancer cells is like policemen catching criminals, locating them by their personal characteristics(biomarkers) and finding them specifically.

Major advantages of Aptamer
| Characteristics | Aptamers | Antibodies |
|---|---|---|
| Immunogenicity | Low | High |
| Internalization | Fast | Low Efficient |
| Modification | Easily Modifiable | Hard to modify |
| Batch-to-batch variation | High Uniformity of Product | Low Uniformity of Product |
| Production & Development Cost | Low | High |
| Production & Development Time | Short | Long |
| Versatility | Wide Range of Targets | Targets with Immunogenicity |
1. Aptamers are not immunogenic and thus are less likely to cause allergic diseases.
2. The production cost of the aptamer is much lower than that of the antibody due to advantages in the
production method and production period.
3. Aptamer is capable of large-scale in vitro screening and production, while antibodies need
mammals to produce which is difficult to scale up and may require complicated modification or cause problems
such as viral contamination.
4. The versatility of aptamer is wider, due to its ability to bind to various targets, including ions,
proteins, and so on. Whereas antibodies only target immunogenic sits.
What Aptamers We chose to use?
Biomarker of Breast Cancer Cells - HER2
The most common breast cancer biomarkers include estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2/Erbb2). HER2 is one of the deepest-researched BC receptors and more than 25% of the BC cases involve HER2 receptors. Human Epidermal Growth Factor Receptor 2(HER2) is the biomarker that our aptamers aim to target.

HER2 is a type of transmembrane glycogen protein that has three different regions. It has an N-terminal extracellular domain (ECD), which further divides into subdomains (I-IV). The subdomain II has a dimerization arm that propagates the dimerization. Pertuzmab and Trastuzumab, two common monoclonal antibody drugs, function as dimerization inhibitors. Their binding to the dimerization arm of HER2 blocks the signal for inhibiting the dimerization with other proteins retarding the cell propagation.
HER2 targeting Aptamer
After rounds of research, we chose to apply two types of the aptamer. HB5 Aptamer [3] and H2 Aptamer[4]. For convenience, we rename HB5 Aptamer to HR2 Aptamer.
Both aptamers are selected through SELEX. H2 Aptamer is used to coat carbon-nanotubes to detect HER2 protein, whereas HR2 Aptamer is design to conjugate with Dox and deliver it to HER2 positive breast cancer cells. According to the literature, H2 Aptamer has a Kd of 270nM and HR2 Aptamer has a Kd of 316nM. We will test both of these aptamers using ELONA and conjugate them onto nanoparticles to help deliver to cancer cells.


Attaching pH-sensitive Motifs to Improve Specificity
As an important part of our project, we plan to add a pH-sensitive motif to the aptamer to further improve the specificity of our drug delivery system.
Since HER2 receptors are also presenting on the surface of some somatic cells, other than breast cancer cells, the wrong targeting of aptamers to the somatic cells might result in serious side effects as the drug killing the normal cells. To deal with this issue, we have searched for other unique characteristics of tumor cells.
Metabolic changes that create a favorable environment for tumor survival, proliferation, and metastasis result in the dysregulation of extracellular and intracellular pH of cancer cells. The extracellular pH for a healthy mammalian tissue is about 7.4, whereas the tumor environment has a lower pH, at 6.7-7.1. Therefore, we aim to engineer our aptamer so that its affinity can decrease dramatically under acidic cancerous conditions, which means that the aptamer will only bind to its target and kill the cell within the tumor environment.
We will introduce a pH-sensitive motif[7][8], whose binding ability increases as the pH decreases, to the aptamer, so that they will only function in the tumor environment, with lower pH, and thus reduce the possible damage of our drug to somatic cells (More in experimental design).
Nanoparticles: PLGA & Liposome
Nanoparticles are small particles that can encapsulate the drug. They are various organic or inorganic coatings that have diverse properties. We aim to load our drug into nanoparticles attached by aptamers[7]. PLGA and liposome, the two nanoparticles, are chosen for our project as they are widely used in encapsulating drugs.
There are many advantages of using NPs for drug delivery:
1. High Stability: the encapsulation for the drugs protect them against enzymatic and hydrolytic degradation as the nanoparticle carriers usually have enhanced solubility, and thus the half-life of drug in blood circulation is prolonged. 2. Lower Side Effect: nanoparticles carrying drugs can specifically accumulate in wounded tissues due to the better permeability and retention effect(EPR). Moreover, as a nano-barrier for the drug, the nanoparticle layer contributes to the slower release of the drug and thus lowering its toxicity. 3. Versatile Uses: It is easy to modify the type of drug it carries, or, we theorize, it can carry multiple types of drugs which only functions when both binds to the target to add another failsafe mechanism to prevent cytotoxic damage against normal tissue cells.

PLGA
Poly D, L-lactide-co-glycolide (PLGA) is an FDA-approved polymer and it is widely used for the delivery of various chemotherapeutic agents to the target site. PLGA is a strong candidate as a drug carrier for a drug delivery system because of its biocompatibility and biodegradability. Moreover, PLGA has high solubility and permeability, enabling it to be stable with slow drug release over a long period of time, thus reducing its side effects and increasing the cytotoxicity for tumor. This makes PLGA an efficient nanocarrier for less hydrophilic anti-cancer agents.
Rapid opsonization by cells of the phagocytic system is a major limitation for achieving effective drug targeting to the site of action by PLGA nanoparticles. Thus, to maximize the therapeutic benefits of drug-loaded nanoparticles they should be able to evade the reticuloendothelial system (RES).[6]
We plan to coat the PLGA surface with chitosan (CS) and polyethylene glycol (PEG), for curbing the phagocytic effects and enhancing the longevity of the nanoparticles.
Chitosan is a biodegradable, biocompatible cationic polymer with low toxicity, mucoadhesive properties, biodegradability and ability to enhance the penetration of large molecules across mucosal surfaces.[2]
Hydrophilic poly (ethylene glycol) (PEG) was introduced as an additional coating polymer to form PEG-coated (PLGA–CS–PEG) nanoparticles mainly because the chemical modification of CS with PEG not only improves the biocompatibility of CS but also reduces the adsorption of circulating plasma proteins onto the material surface. PEG-coated nanoparticles have been found to be of great potential in shielding the nanoparticles from the RES due to steric repulsion resulting from a loss of configurational entropy of the bound PEG chains and their rapid motion in aqueous media. In addition, hydrophilic PEG can form a hydrated outer shell, thereby protecting the nanoparticles from being quickly uptaken by the RES, thus extending the half-life of drugs.
Liposome
Liposomal NPs (LNPs) are spherical vesicles that are formulated by incorporating one or more phospholipid bilayers and their size can reach up to a few hundred nanometers. The LNPs contain a hydrophilic inner core which is surrounded by the hydrophobic lipid bilayer. Because of this unique morphology, either hydrophilic, hydrophobic, or amphiphilic drugs can be encapsulated.[5]
One of the major drawbacks of the liposomal formulation was its rapid clearance from blood due to the adsorption of plasma protein to the phospholipid membrane of the liposomes, thereby triggering the recognition and uptake of the liposomes by the mononuclear phagocytic system (MPS). Fortunately, when the surface of the liposomes was modified with a flexible hydrophilic polymer such as polyethylene glycol (PEG), the uptake by MPS could be retarded. This resulted in a significant increase in the biological half-life. Therefore, we will coat liposomes with PEG to increase stability.

EPR Effect
Blood vessels passing tumor tissues have presented more defective architecture due to the excessive needs of oxygen and nutrients by cancer cells. This enhanced the probability for nanoparticles sized between 150-200nm to be trapped inside these tissues, without significantly damaging normal tissues. Therefore we chose to use liposomes sized 200nm to carry the drug.[9]
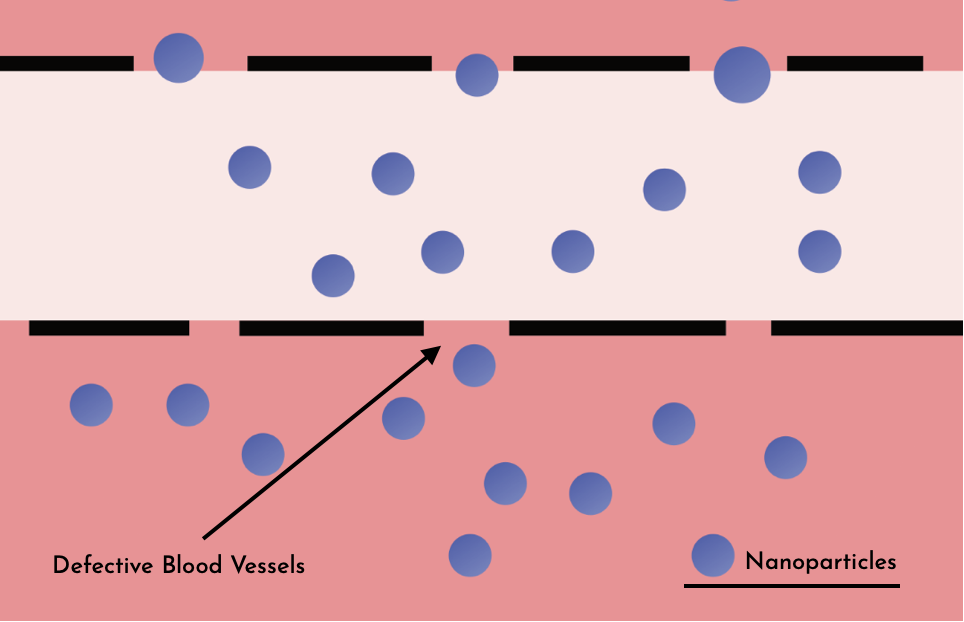
Nanoparticles can be internalized by tumor cells by phagocytic processes followed by endosomal escape and delivery of encapsulated agents to the cytosol.
Doxorubicin
Doxorubicin, a chemotherapeutic agent with strong activity against a wide range of human malignant neoplasms, is the drug we applied in our nanoparticle. It is a type of chemotherapy drug also known as 'Anthracycline'.

There are mainly two proposed mechanisms by which doxorubicin acts in the cancer cell: Disruption of topoisomerase ll mediated DNA repair; and free radical formation, both routes trigger apoptotic pathways of cell death It has been in use as a key anticancer drug for forty years and is thus well-researched.
We choose doxorubicin (DOX) as the drug contained by our nanoparticle since it is one of the most promising and deepest-researched treatments for early and advanced breast cancer; DOX has been in use as a key anticancer drug for forty years. However, the application of doxorubicin is limited by its adverse effects such as severe cardiotoxicity. Therefore, we spend lots of time improving our system’s specificity and stability to avoid the side effects resulting from doxorubicin leakage.
Summary of Whole System
In summary, we designed a bioconjugate that uses specific aptamers to functionalize nanoparticles for actively targeting for breast cancer cells. Functionalized nanoparticles are sphere-shaped vesicles in an aqueous solution. Its hydrophilic groups form a hydrophobic core inside which provides the foundation for the encapsulation of Dox. And its hydrophobic tails assemble shoulder by shoulder to form a shell-like membrane structure which is the basis for the stability of nanoparticles. In the outermost layer of the nanoparticles, hydrophilic aptamers are conjugated to their surface with amido linkage. The protruding aptamers mediate the targeting to HER2 receptors with high specificity and affinity. All of our efforts aim to stably and efficiently deliver anticancer drugs to cancer cells.
Moreover, to enhance the efficacy and reduce the toxicity of our design, we plan to make some modifications to both aptamers and nanoparticles. For example, we will try to modify our aptamers to make them pH-sensitive. We will try our best to improve the therapeutic efficacy of the targeted drug delivery system, by destroying the tumor cells at a maximum, while bringing little damage to healthy tissue cells.
If you would like to see what experiments we conducted in order to achieve our design, go to our Experimental Design Page.
If you would like to see our results to the experiments go to Results Page.
Reference
- Hongguang, S., Jingsan, Z., Jianbo, W., Youli, Z. & Zhu, X. (2016) Aptamer Technology and Its Application In Tumor Diagnosis and Therapy. Prog Pharm Sci Aug. 2016 Vol. 40 No. 8.
- Parveen, S., & Sahoo, S. K. (2011). Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. European journal of pharmacology, 670(2-3), 372–383. https://doi.org/10.1016/j.ejphar.2011.09.023
- Liu, Z., Duan, J. H., Song, Y. M., Ma, J., Wang, F. D., Lu, X., & Yang, X. D. (2012). Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. Journal of translational medicine, 10, 148. https://doi.org/10.1186/1479-5876-10-148
- Niazi, J. H., Verma, S. K., Niazi, S., & Qureshi, A. (2015). In vitro HER2 protein-induced affinity dissociation of carbon nanotube-wrapped anti-HER2 aptamers for HER2 protein detection. The Analyst, 140(1), 243–249. https://doi.org/10.1039/c4an01665c
- de Sousa Marcial, S.P., Carneiro, G. & Leite, E.A. Lipid-based nanoparticles as drug delivery system for paclitaxel in breast cancer treatment. J Nanopart Res 19, 340 (2017). https://doi.org/10.1007/s11051-017-4042-0
- M. Hashemi, A. Shamshiri, M. Saeedi, L. Tayebi, R. Yazdian-Robati, Aptamer conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs, Archives of Biochemistry and Biophysics (2020), doi: https://doi.org/10.1016/j.abb.2020.108485.
- Thompson, I., Zheng, L., Eisenstein, M., & Soh, H. T. (2020). Rational design of aptamer switches with programmable pH response. Nature communications, 11(1), 2946. https://doi.org/10.1038/s41467-020-16808-2
- Idili, A., Vallée-Bélisle, A., & Ricci, F. (2014). Programmable pH-triggered DNA nanoswitches. Journal of the American Chemical Society, 136(16), 5836–5839. https://doi.org/10.1021/ja500619w
- Maeda, H., Nakamura, H., & Fang, J. (2013). The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Advanced drug delivery reviews, 65(1), 71–79. https://doi.org/10.1016/j.addr.2012.10.002
- Gao, W., Thamphiwatana, S., Angsantikul, P., & Zhang, L. (2014). Nanoparticle approaches against bacterial infections. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 6(6), 532–547. https://doi.org/10.1002/wnan.1282


