Background
Exposure to prolonged chronic stress induces heightened vulnerability to anxiety, depression, and a lot of mood disorders. Dysfunction of the medial prefrontal cortex (mPFC) in the brain has been linked to the cognitive and emotional deficiency induced by long-term stress exposure. Chronic stress-induced depression (CSID), therefore, is an imperative mental disorder. If treated improperly, upcoming psychiatric disorders, such as major depressive disorders (MDD), anxiety, and even post-traumatic stress disorder (PTSD) caused by lasting sensitivity to recurring sensors, will eventually tear the patient to shreds[1]. According to the World Health Organization (WHO), depression is already becoming the leading cause of disability around the world. It even haunts more than 264 million people worldwide[2]. However, it is only the tip of the iceberg.
Depression is a complex disease. Deficits of neurotransmitters, genetic inheritance, and dysregulation of the hypothalamus-pituitary-adrenal axis (HPA axis), etc, widely account for depression. Nevertheless, the nature of this disorder has recently been reconceptualized as dysregulation of the gut-brain axis. Mounting scientific evidence indicates that gut-modifying agents could regulate intestinal function and health, which is widely composed of activated immune-inflammatory substances and oxidative stress (OS)[3]. Those pathways mentioned above will be further discussed in the following sections.
Immune-inflammatory Pathway in Depression
Interferon-gamma (IFN-γ) and other pro-inflammatory Th1 cytokines are known to be positively correlated with depression-like behavior and trigger sickness behavior[4]. Lots of evidence indicate that the activation of immune-inflammatory pathways and neural-immune interactions are intimately involved in the pathophysiology of depressive disorders. There are two major findings that signify immune-inflammatory pathways[3].
Higher serum levels of inflammatory cytokines
interleukin-6 (IL-6), IL-1β, and TNF-α could cause depressive disorders by disrupting neurotransmitter synthesis and signal transduction.
T-cell abnormal activation
elevated serum levels of neopterin, as well as activation of Th1-like and Th-17-like cells, including increased production of IL-2 and IFN-γ.
Though the problem is calamitous, taurine metabolites could decrease the excessive amount of inflammatory cytokines. As for IFN-γ, it could be a potential biomarker for preliminary CSID.
Oxidative Stress Pathway in Depression
The intracellular biological imbalance between ROS (reactive oxygen species) and antioxidants, leading to the alteration of biomolecules and the dysregulation of redox signaling pathways, is known to be OS. Both neuronal OS and intestinal OS could lead to the occurrence of CSID. Followings are physiological damages inflicted by excessive ROS[3]:
- Oxidative stress with its derivatives can affect neuronal signaling and functions, causing damage to vital intracellular macromolecules such as DNA and proteins. Therefore, excessive oxidative stress will induce inflammation of neurons, which leads to abnormal brain function in patients with depression[5].
- Chronic intestinal inflammation is strongly related to increased ROS, including superoxide, hydrogen peroxide, and hydroxyl radical. It will indirectly lead to depressive symptoms by the gut-brain axis if treated improperly. The importance of intestinal ROS is emphasized by the massive production of Myeloperoxidase (MPO) by neutrophils, serving as a strong biomarker for gut inflammation clinically; and increase the level of NADPH oxidase, yielding OS far beyond normal range.
Those devastating injuries are hard to navigate and control. Nevertheless, taurine metabolites could precisely target those problems
Taurine
Taurine is a semi-essential inhibitory neuromodulatory amino acid appreciated for its role in neural development and neurogenesis as a neuroprotective agent. In chronically stress-induced depressive mice models, taurine dietary has increased the L-arginine concentrations in the hypothalamus with a significant decrease in the duration of immobility in forced swimming tests[6].
Moreover, studies have shown that taurine with its metabolites could significantly reduce the level of both reactive oxygen species such as HOCL and IFN-γ. Further information will be discussed in the Model part.
These results strongly suggest its capability in reducing stress-induced depressive behaviors.
Approaches
Menble
Menble is a special edible bubble that aims to alleviate someone’s depression by producing taurine based on sensing IFN-γ and oxidative stress biomarkers by our functional engineered E. coli Nissle 1917 bacteria. This way, the amount of taurine produced by our bacteria is based on the stress level in the gut. Although energy drinks also contain taurine, the amount of taurine in each serving is fixed, and could not sense the level of stress that is present in the body. Not only that, there are also reports of complications from people with kidney problems after consuming more taurine than needed[7,8], therefore it is important for our bacteria to produce taurine based on a person’s stress level. By understanding the nature of a stressed, depressive-like community, that is most sufferers don’t seek medical help due to stigma[9,10], and even then patients rarely adhere to the medications prescribed by their doctor [9]
, our product must be affordable, attractive, and easily accessible to increase adherence from patients and reach the ones who can’t seek medical help. We have selected bubble tea, a signature drink representing Taiwan that is low-cost and popular all around the world. This sweet delicious drink is enjoyed by all walks of life. Therefore, we can incorporate this medication into everyone’s daily life, without having to regularly take medications that must be prescribed by a doctor. How Menble works is it packages our functional E. coli Nissle 1917 which contains plasmid with our taurine-producing biobrick on it, and protects it from degradation by gastric acid in the stomach[11,12]. Its alginate composition also allows it to be digested in the intestine where the environment is basic enough, and also the combined action by acid and trypsin is able to break down its structure[12]
, releasing the functional bacteria into the intestine. After our bacteria produces taurine induced by the IFN-γ and oxidative stress biomarkers present in the intestine, taurine can then be transported to the brain via the gut-brain axis, making people happy again!
f(int)
In order to predict how Menble will behave within our intestine, logically thinking, a human clinical trial is usually performed; nevertheless, due to animal experiments regulations and medical ethics, it is more accessible and convenient for us to perform simulation if we build an in vitro platform which can represent the human's intestine. Consequently, we decided to build f(int), a microfluidic chip able to imitate the environment of a human jejunum and determine the residual rate of E. coli Nissle which stays inside the guts.
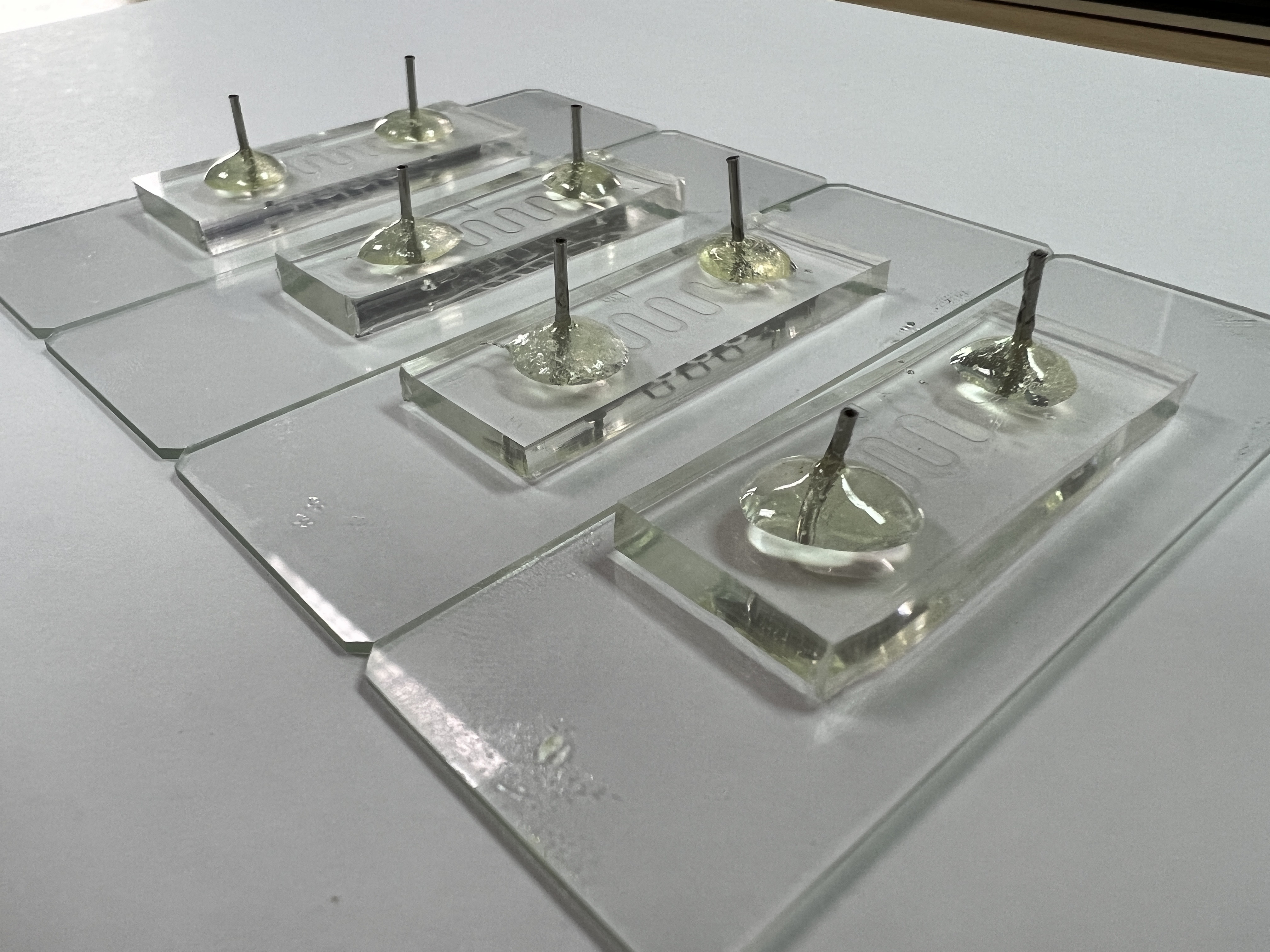 Fig. 1. f(int) intestinal microfluidic chip
Fig. 1. f(int) intestinal microfluidic chip