Overview
In recent years, the development husbandry in China has been preoccupied with great
opportunities. Still, the energy feed is relatively lacking, which makes animal production development face
severe supply and demand challenges in the future. Silage has been a good choice for ruminants feeding,
whereas, the cellulose contained in silage causes potential problems in ruminants’ digestive system. To
address this problem, we are planning to develop feeding additives containing cellulase, which will be
compatible with silage, thereby enhancing ruminants’ digestion. With this efficient uptake of feed, the
feeding costs can be reduced while improving the yield and quality of ruminants.
In our design, the cellulase gene is extracted from Pseudomonas aeruginosa, P. aeruginosa, PKC-001. The gene
is connected to vector pET-25b and then transformed to E.coli (BL21) and to construct plasmid pET25b-PKC-OP.
The engineered E.coli is used to test protein expression conditions, and then used to conduct enzyme
activity test, e.g. the degrading capacity of our engineered strain of cellulose.
In the future plan, the E.Coli(BL21) will be replaced by other probiotic, such as
Nissle 1917 to make further test about protein expression and enzyme activity.
Supporting Experiment Results
 Figure 1 E. coil having the desired pET-25b and PKC-OP
Figure 1 E. coil having the desired pET-25b and PKC-OP
The pET25b-PKC-OP was constructed.
The plate shows monoclonals of pET25b-PKC-OP constructs
 Figure 2. The result of Apa1 enzyme digestion validation
Figure 2. The result of Apa1 enzyme digestion validation
Column “marker” is a column that is used to show the position of different lengths
of genes. Number 1 to 10 is the result for recombinant plasmid pET25b-PKC-OP after Apa1 enzyme digestion. We
get two bands of 4712bp and 1894bp. It further indicates that the obtained monoclonals were positive
monoclonals containing the recombinant plasmid.

 Figure 3. SDS-PAGE analysis of culture supernatant under different induction conditions
Figure 3. SDS-PAGE analysis of culture supernatant under different induction conditions

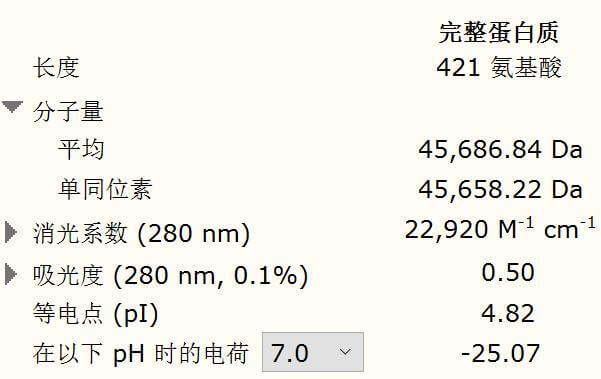 Figure 4. Protein molecular weight result of PKC-op by Snapgene
Figure 4. Protein molecular weight result of PKC-op by Snapgene
We designed several different induction conditions for protein expression. As the
vector we chose to construct the plasmid contains a pelB signal peptide, the engineered strain would secret
the protein into the medium. Therefore, we collected the culture supernatant after induction and ran
SDS-PAGE for verification.
According to the calculation by Snapgene, the expected protein molecular shall
weigh 45.6 KDa. As seen from the SDS-PAGE map (Fig. 4), there is a wide band just above 40 KDa and it
indicates that we have obtained the PKC-op protein.
Our experiment proved the potential success of our design, e.g. the PKC-op protein
could be acquired by genetic engineering on the chassis of E.coli (BL21). In the future, we will move on
with the experiment to test the protein’s degradation capacity of cellulose, consolidating the research
foundations for future development of our feeding additives.

