
Overview
We followed the engineering cycle for countless iterations over the course of our project. For clarity, we have condensed the iterations and dispersed our experience into the steps:
1. Background Investigation and Information Collection
2. Design
3. Build and Test
4. Model, Learn and Improve

Background Investigation and Information Collection
1. Overview
2. Macrophage and SARS-CoV-2
3. Macrophages, Cytokines, and Cytokine Storm
4. Macrophage Phenotype and Disease
5. Artificial Cell Therapy and Artificial Macrophage Therapy
Before designing our toggle macrophages, we conducted a thorough literature review on the important role of macrophages in response to COVID-19. According to literatures, Macrophages are divided into M1 (classically activated) and M2 (alternatively activated) macrophages, which are responsible for suppressing infection and regulating inflammation respectively. Through the analysis of the existing artificial cell therapies, we find the possibility in modifying the macrophages to alter their performances in phagocytosis and cytokine expression.
The findings of our literature review are summarized below.
A novel coronavirus, severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), which emerged in 2019, has been found to cause acute respiratory distress syndrome (ARDS) called coronavirus disease 2019 (COVID-19), with some patients developing a severe form of this disease leading to death. This virus is the third highly pathogenic coronavirus reported to date and has caused a worldwide outbreak of infection, a pandemic. Around 80% of individuals infected with SARS-CoV-2 appear to recover without antiviral treatments (Wu Z et al.2020), indicating that an adequate host immune response against the virus may be sufficient to cure the viral infection. In contrast, the other 20% of patients develop severe forms of this disease, suggesting that an inadequate immune response may predispose patients to poorer outcomes. It has been reported that some strains invade the body in a dormant and a mitigatory way. At the beginning of the infection, SARS-CoV-2 can not only suppress the innate immune response by inhibition of interferon Ⅰ mediated inflammatory cells recruitment, but it can also escape the murder of acquired immune cells by lowering the expression of surface antigen. And the virus can consequently get mass propagation in alveolar epithelia cells without the worry of being destructed by the immune response.

Understanding host immune responses against SARS-CoV-2 may be important in designing therapeutic strategies to fight viral infection. (Mallis P et al.2020) Macrophages, as an important component of the innate immune system, have unique effector functions as a first-line defense against SARS-CoV-2.
1. Macrophages participate in the innate immune response and play a critical role in the body’s defense against viral infections. They are powerful activators for triggering adaptive immune responses and killing viruses (Meidaninikjeh et al. 2021).
2. Naturally, they can transform into M1 type and M2 type, which is pro-inflammatory and anti-inflammatory, respectively. This characteristic of macrophages corresponds to our ideal therapy that can concurrently eradicate the viruses and balance the immune response.
3. Delayed IFN-I release is associated with inactivation of macrophages, which later causes delayed viral clearance and increased proinflammatory cytokine release.
Therefore, we hypothesized that we could utilize macrophages in a synthetic way to combat SARS-CoV-2.

Cytokine, mainly produced by immune cells, is a kind of highly active small molecule polypeptide or protein. The network formed by the interactions among cytokines plays an essential role in immune response, inflammatory response, and cell proliferation and growth. (Yao Liteng et al.2020)
In the inflammatory response, a variety of cytokines exert their physiological functions and regulate the immune response when binding to their specific receptors. Some cytokines equipped with the capability of activating immune cells and promoting inflammatory response are called pro-inflammatory cytokines; while the other cytokines which could resist inflammation are called anti-inflammatory cytokines. When our body is invaded by pathogens, the pro-inflammatory cytokines produced by the immune system promote the progression of inflammation and elimination of the pathogens; and the anti-inflammatory cytokines inhibit the inflammatory response. The two sides work together, thus presenting a complex immune response.
It has been suggested that pathophysiology of SARS-CoV-2 is related to elevated levels of inflammatory cytokines such as IL-2, IL-6, IL-7, GSCF, IP10, MCP1, MIP1A, and TNF-α, an event which is known as “cytokine storm” (Mehta P et al.2020; Law HK et al.2005). After the infiltration of SARS-CoV-2 to the host cells, infected patients would go through three-phase immunopathology—non-severe stage, replication cycle and prolonged lung damage. It is right in the third stage that “cytokine storm” has a high association with disease severity and mortality (Mallis P et al.2020). We therefore concluded that the key to preventing the progression of COVID-19 is to regulate the expression of cytokines. And the macrophage is the center of the cytokine storm to some extent (Chau AS et al.2021).

Picture from: Chau AS, Weber AG, Maria NI, Narain S, Liu A, Hajizadeh N, Malhotra P, Bloom O, Marder G, Kaplan B. The Longitudinal Immune Response to Coronavirus Disease 2019: Chasing the Cytokine Storm. Arthritis Rheumatol. 2021 Jan;73(1):23-35. doi: 10.1002/art.41526. Epub 2020 Dec 1. PMID: 32929876.
Macrophages are distinguished to M1 (classically activated) and M2 (alternatively activated) macrophages (Yagi H et al.2010). M1 macrophages are responsible for pathogen phagocytosis and presentation of antigen epitopes to dendritic cells (DCs). In this process, a set of inflammatory cytokines are produced by M1 macrophages, such as TNF-α, ΙL-1α/β, IL-6, and IL-12, thus activating and promoting Th1 responses. On the other hand, M2 macrophages promote Th2 responses and are considered as immunosuppressive cells. These cells exhibit low expression of inflammatory cytokines and high production of anti-inflammatory IL-10, which is associated with tissue remodeling, wound repair, and clearance of apoptotic cells (Shapouri-Moghaddam A et al.2018).

M1 and M2 macrophages have different functions and transcriptional profiles. They have unique abilities by destroying pathogens or repairing the inflammation-associated injury. It is known that M1/M2 macrophage balance polarization governs the fate of an organ in inflammation or injury. When the infection or inflammation is severe enough to affect an organ, macrophages first exhibit the M1 phenotype to release TNF-α, IL-1β, IL-12, and IL-23 against the stimulus. But, if M1 phase continues, it can cause tissue damage. Therefore, M2 macrophages secrete high amounts of IL-10 and TGF-β to suppress the inflammation, contribute to tissue repair, remodeling, vasculogenesis, and retain homeostasis. (Shapouri-Moghaddam A et al.2018).
Based on this, we designed the toggle macrophages which could cover the whole progression of the infection. In the early stage, the macrophages would transform into M1 phenotype, increasing the expression of the cytokines to eliminate immunosuppressive as much as possible. When the infection deteriorates into the late stage, the toggle macrophages would transform into M2 phenotype, exhibiting low levels of cytokine expression to decrease its critical damage to the severely ill patients.
For more detailed information, please visit our Design.
5.1 CAR-T cell therapy
In recent years, Chimeric antigen receptor (CAR)-engineered T cells (CAR-T cells) have yielded unprecedented efficacy in the treatment of tumor, especially B cell malignancies (Wang Z et al.2017).
Chimeric antigen receptor (CAR) is a modular fusion protein comprising extracellular target binding domain usually derived from the single-chain variable fragment (scFv) of antibody, spacer domain, transmembrane domain, and intracellular signaling domain containing CD3ζ linked with zero or one or two costimulatory molecules such as CD28, CD137, and CD134 (Eshhar Z et al.2008; Curran KJ et al.2012; Dai H et al.2016). T cells engineered to express CAR by gene transfer technology are capable of specifically recognizing their target antigen through the scFv binding domain, resulting in T cell activation in a major histocompatibility complex (MHC)-independent manner (Eshhar Z et al.1993).
However, on-target or off-target tumor toxicity resulting from the recognition of healthy tissues by CAR-T cells would cause severe and even life-threatening toxicities. What’s worse, the activation of T cells is always accompanied by the overproduction of cytokines, which suggests that the application of CAR-T cells in the treatment of COVID-19 is impractical. But at least, it provides with an idea of constructing CAR.
5.2 CAR-Macrophage therapy
As mentioned above, CAR-T cell therapy is not so effective in combating solid tumor. Given that macrophages are the innate immune cells with the highest infiltration rate in the tumor microenvironment, researchers are trying to use macrophages modified with CAR (CAR-M) against solid tumors.
CAR-M with intracellular domain of MEGF10 or FcRγ, promotes the phagocytic potential of target antigens. (Chen Y et al.2021)
So, we began to think about whether we could modify macrophages with different CARs to alter the performance, such as a strong phagocytosis and a better control of cytokine expression.
Design
1. CAR Receptors
2. IL-6 Sensor and Linked Circuit Design(more details in Proof of Concept)
We engineered THP-1 cells with 5 kinds of CAR to enhance its recognizing ability and phagocytosis of SARS-CoV-2. They share similar constructions in extracellular domain and transmembrane domain, except for intracellular domain. Notably, the Myc tag is inserted for flow cytometer.
1. The extracellular domain: a single chain variable fragment (ScFv) which specifically recognizes the spike protein of SARS-CoV-2.
2. The transmembrane domain: CD8 protein allowing signal transduction.
3. The intracellular domain: a domain consists of different signal motifs which stem from the intracellular structure of various natural receptors of immune cells. These signal motifs can trigger robust phagocytosis. Noteworthy, we designed a CAR without intracellular structure as negative control.
4. Myctag is a fluorescent protein gene for subsequent detection of CAR macrophage transduction.
Why we chose these four signal motifs as candidates? According to research, MERTK is a tyrosine kinase which belongs to TAM family of RTKs. The activated TAM receptors can inhibit the TLR and type I IFN signaling pathways and thus may avoid further upregulation of proinflammatory cytokine levels (Todt, Hu, and Curtis 2004). In previous study, Megf10 and FcRγrobustly triggered engulfment of CAR-macrophages by being phosphorylated by Src family kinases, while CD3ζ by recruiting syk kinase (Morrissey et al. 2018).

Figure1:Vector maps of tested CAR designs and schematics showing the structures of CARs
According to the in vitro experiments,CARγ macrophage (Mγ) can promote inflammation and CARMERTK-macrophage (Ms) can inhibit inflammation. We determined to integrate CARγ and CARMERTK into a single macrophage by our circuit design in order to construct the smart Toggle Macrophage which can respond to the changing microenvironment by switching its phenotype. The circuit we designed determines the function of Toggle Macrophage by regulating the CAR expression based on the IL-6 concentration in the microenvironment. The circuit is as followed:
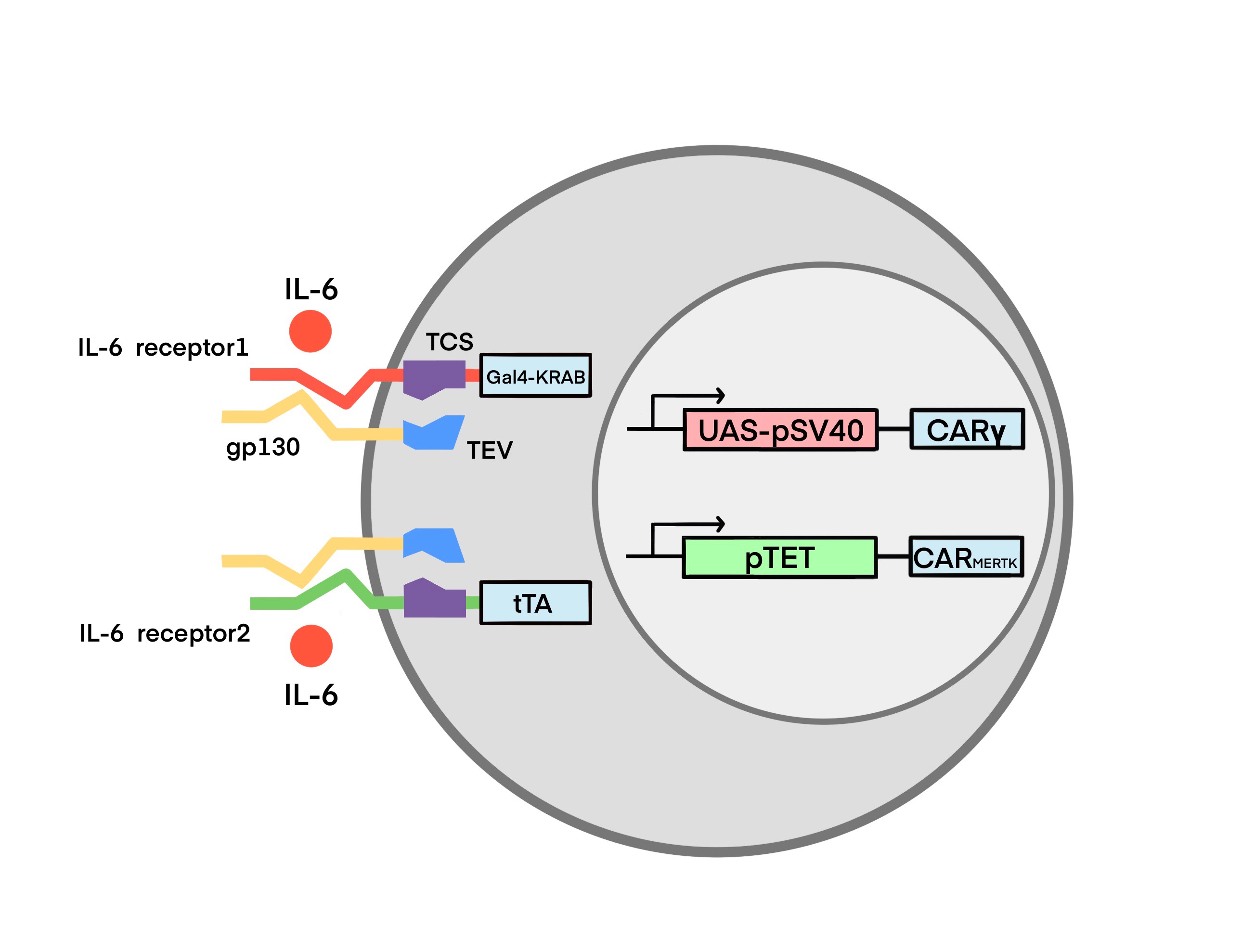
(figure2: Circuit Design of Toggle Macrophage)
Build and Test
We aimed to test the 4 kinds of CAR macrophages we constructed. To accomplish this, we conducted several experiments to test their engulfment effect, phenotypic transformation and the capacity of viral load. Scroll down to learn more.
We designed 7 basic parts synthesized by GENEWIZ to synthesize 4 composite parts.
| Name | Type | Description | |
|---|---|---|---|
| Tag | BBa_K4040005 | Tag | Myc tag |
| Extracellular domains | BBa_K4040019 | Coding | CR3022 scFv |
| Transmembrane domains | BBa_K4040004 | Protein_Domain | CD8 hinge |
| BBa_K4040007 | Protein_Domain | CD8TM | |
| Intracellular domains | BBa_K4040000 | Protein_Domain | Intracellular Domain of the MEGF10 Protein |
| BBa_K4040001 | Protein_Domain | Intracellular Domain of the MERTK Protein | |
| BBa_K4040002 | Protein_Domain | FcRγ | |
| BBa_K4040003 | Protein_Domain | CD3 zeta chain | |
| Composite part | BBa_K4040015 | Composite | CAR-MEGF10 |
| BBa_K4040016 | Composite | CARγ | |
| BBa_K4040017 | Composite | CAR ζ | |
| BBa_K4040018 | Composite | CAR-MERTK |
3.0 Method
Cell Lines and Primary Human Cells
All cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The identities of the cell lines were verified by STR analysis, and the cell lines were confirmed to be mycoplasma free. 293 and Vero cells were maintained in DMEM supplemented with 10% fetal bovine serum, and THP-1 cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. Cell culture media and supplements were obtained from Life Technologies, Inc.
Vector Construction
The sequence encoding the scFv generated from CR3022 was chemically synthesized. As shown in Figure 1, synthetic receptors contained the human CD8a signal peptide followed by the scFv linked in-frame to the hinge domain of the CD8a molecule, transmembrane region of the human CD8 molecule, and intracellular signaling domains of the FCER1G, MEGF10, MERTK or CD3z molecules. The cDNA sequences containing the various fusion constructs were cloned into a third-generation lentiviral vector in which the CMV promoter was replaced with the EF-1a promoter, the pELNS vector. (Carpenito C et al. 2009) High-titer replication-defective lentiviruses were produced and concentrated (Carpenito C et al.). Lentiviral infection was used to stably express CAR constructs in THP-1 cells.
FACS-Based Phagocytosis Assay
UTD or CAR-expressing THP-1 cells were cocultured with GFP+ 293T cells or GFP+ 293T-S (S+ ) target cells for 4 h at 37°C. The effector-to-target (E:T) ratio was 1:1, and 1 × 105 cells were used as both effector cells and target cells. After coculturing, the cells were harvested and stained with an antiCD11b APC-Cy7-conjugated antibody (M1/70, BioLegend) and analyzed by FACS using a FACSCalibur flow cytometer (BD Biosciences). The percentage of phagocytosis was calculated based on the percent of GFP+ events within the CD11b+ population. Data are represented as the mean ± standard error of quadruplicate wells.
Flow Cytometry
Cell-surface staining was performed for 45 min at 4°C and was analyzed using a FACSCalibur flow cytometer (BD Biosciences). A minimum of 1 × 104 events per sample were examined.
In Vitro Cytotoxicity Assay and Luciferase-Based Killing Assay
293T and 293T-S cells were used as targets in luciferase-based killing assays including control (UTD) or CAR macrophages. The effector-to-target (E:T) ratio was 10:1 for all the groups. Bioluminescence was measured using a Bio-Tek Synergy H1 microplate reader. The percent specific lysis was calculated on the basis of the experimental luciferase signal (total flux) relative to the signal of the target alone, using the following formula: %Specific Lysis = [(Sample signal —; Target alone signal)]/ [Background signal — Target alone signal)] × 100.
SARS-CoV-2 Pseudovirus and Cell Infection Experiments
The SARS-CoV-2 pseudovirus was constructed based on the spike genes of the strain Wuhan-Hu-1 (GenBank: MN908947) using published methods (15). The SARS-CoV-2 spike gene was chemically synthesized and cloned into a eukaryotic expression plasmid. 293T cells were first transfected with the S expression vector and then infected with a VSV pseudotyped virus (G∗DGVSV), in which the VSV-G gene was substituted with luciferase expression cassettes. The culture supernatants were harvested and filtered at 24 h post infection. The SARS-CoV-2 pseudovirus could not be neutralized by anti-VSV-G antibodies, and no G∗DG-VSV was mixed with the SARS-CoV-2 pseudovirus stock. For cell-based infection assays, target cells were grown in plates until they reached 50%–75% confluency and were then inoculated with pseudotyped virus. The transduction efficiency was quantified at 16 h post transduction by measuring firefly luciferase activity according to the manufacturer’s instructions (Promega).
Phagocytosis Assay
In all cases, SARS-CoV-2 S pseudotyped virions were pelleted (90 min at 14,000 rpm and 4°C), and after removal of the supernatant, the pellets were resuspended in RPMI medium and incubated with phagocytes (THP-1 cells or CAR macrophages) at 37°C for 1.5 h. After allowing time for phagocytosis, the cells were washed three times with PBS and incubated with Accutase (Innovative Cell Technologies) for 10 min at 37°C, followed by a final wash in Accutase. Intracellular staining for the S protein was performed for 60 min on ice after using a fixation/permeabilization kit (eBioscience) and then analyzed using a FACSCalibur flow cytometer (BD Biosciences). The phagocytic score was determined by gating the samples on events representing cells and was calculated as follows: Percent S protein positive × median fluorescence intensity (MFI).
Cytokine Analysis
Cytokine analysis was performed on supernatants derived from cultures given the indicated treatments using a human cytokine 10-plex panel (Thermo Scientific) per the manufacturer’s instructions, with the panel results read on a Luminex Analyzer.
Statistical Analysis
Unless otherwise specified, Student’s t test was used to evaluate the significance of differences between two groups, and ANOVA was used to evaluate differences among three or more groups. Differences between samples were considered statistically significant when P < 0.05.
3.1 engulfment effect test
The phagocytic potential of human macrophage THP-1 cell lines expressing different CAR receptors or a truncated CAR receptor (CARΔ) lacking the intracellular domain was measured with a cell-based assay. The results are demonstrated in figure 1 and figure 2, both of which suggested that CAR macrophages and control untransduced (UTD) macrophages did not show notable phagocytosis of 293 cells; however, CARMEGF10, CARγ and CARζ cells phagocytosed Spike-bearing 293 cells in an S-specific manner.

Figure 3. Phagocytosis of 293T cells

Figure 4. Phagocytosis of 293T-S cells
Antibody-mediated phagocytosis and internalization of virions are important mechanisms of antiviral activity performed by macrophages against pathogens; however, using the phagocytosis assay developed for SARS-CoV-2, we observed low levels of phagocytic activity when UTD cells directly contacted virions. Phagocytic activity was not significantly increased when CAR△ cells rather than UTD macrophages were the phagocytes in the assay, suggesting that the extracellular domain of the CAR alone is not sufficient to induce strong virion internalization. CARγ, CARMEGF10, and CARζ mediated similar significantly stronger levels of SARS-CoV-2 phagocytosis by THP-1 cells than CAR△(truncated CAR).

Figure 5. The uptake of pseudotyped virions by UTD and other CAR macrophages
3.2 Phenotypic test
Because the systemic cytokine profiles observed in patients with severe COVID-19 show similarities to those observed in patients with macrophage activation syndrome, culture supernatants from THP-1 cells with different CARs treated with virions were further analyzed in a multiplex cytokine assay (Figure5). Following SARS-CoV-2 treatment of THP-1 cells, we observed slightly increased secretion of the cytokines IL-6, IL-8 and TNF-a, but no discernable patterns could be confidently drawn for GM-CSF, IL-1b, IL-2, IL-4, IL-5, IL-8, IL-10, and IFN-g. CAR△ cells showed a cytokine profile similar to that of UTD macrophages. Notably, we observed not only strongly increased induction of IL-6, IL-8 and TNF-a but also induction of IFN-g and IL-10 in SARS-CoV-2-treated CARγ and CARζ cells. However, for CARMERTK cells, we did not observe significant changes in cytokines.

Figure 6. Cell lines were infected with the SARS-CoV-2 pseudotyped virus or mock infected. Cytokine levels in the supernatants were determined by a multiplex bead array.
3.3 Viral load test
We further used a transwell-based coculture model to evaluate the protective role of CAR macrophages in SARS-CoV-2 infection (Figure 7). All the CAR-expressing macrophages potently inhibited Vero E6 cell infection with the SARS-CoV-2-S pseudotyped virus. Interestingly, CAR△ cells showed no protective effect in the infection assay, although they had a similar capacity to bind to the S protein, suggesting that the intracellular signaling domain is necessary for virion clearance by CAR macrophages (figure 8).
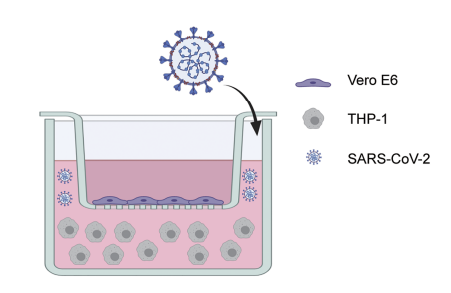
Figure 7. Transwell coculture model
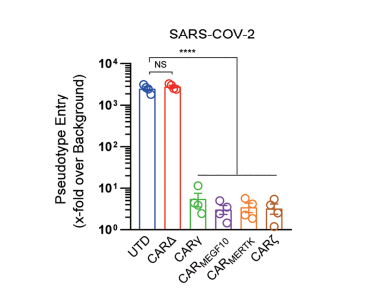
Figure 8. Pseudotype entry intodifferent cell cocultures
After testing, we found that although cytosolic domains from MERTK (CARMERTK) did not trigger antigen-specific cellular phagocytosis or killing effects, unlike those from MEGF10, FcRγ and CD3zeta did, these CARs all mediated similar SARS-CoV-2 clearance in vitro. Notably, we showed that CARMERTK macrophages reduced the virion load without upregulation of proinflammatory cytokine expression. These results suggest that CARMERTK drives an ‘immunologically silent’ scavenger effect in macrophages and paves the way for further investigation of CARs for the treatment of individuals with COVID-19, particularly those with severe cases at a high risk of hyperinflammation. While the other three CARs reduced the virion load with upregulation of proinflammatory cytokine expression.
Model, Learn and Improve
We have expanded upon our Silver medal work for Proposed Implementation and developed a Proof of Concept for our project.
Reference
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of Large, Established Tumor Xenografts With Genetically Retargeted Human T Cells Containing CD28 and CD137 Domains. Proc Natl Acad Sci USA (2009) 106:3360–5. doi: 10.1073/pnas.0813101106
Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Establishment and Validation of a Pseudovirus Neutralization Assay for SARS-CoV-2. Emerg Microbes Infect (2020) 9:680–6. doi: 10.1080/22221751.2020.1743767
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239-1242 [PMID: 32091533 DOI: 10.1001/jama.2020.2648]
Mallis P, Michalopoulos E, Chatzistamatiou T, Stavropoulos-Giokas C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J Stem Cells. 2020 Aug 26;12(8):731-751. doi: 10.4252/wjsc.v12.i8.731. PMID: 32952855; PMCID: PMC7477656.
Merad M, Martin JC.Nat Rev Immunol. 2020 Jun;20(6):355-362. doi: 10.1038/s41577-020-0331-4. Epub 2020 May 6.
Xu Na, Lv Yingfeng. Research progress of macrophages in the immune microenvironment of lung cancer[J]. Journal of Clinical Oncology, 2021, 26(08): 756-761.
Sui Yang, Zhao Zhiyu, Wu Changjun. Research progress of tumor-associated macrophages in triple-negative breast cancer[J/OL]. Chinese Journal of Immunology: 1-11[2021-10-04]. http: // kns. cnki. net/ kcms/ detail/ 22.1126.r.20210624.1517.008.html.
Yao Liteng, Min Jianping, Su Haixiang. The mechanism of cytokines in new coronavirus pneumonia and their monitoring value[J]. Gansu Medicine, 2020, 39(02): 148-150+157.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033-1034 [PMID: 32192578 DOI: 10.1016/S0140-6736(20)30628-0]
Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine upregulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005; 106: 2366- 2374 [PMID: 15860669 DOI: 10.1182/blood-2004-10-4166]
Mallis P, Michalopoulos E, Chatzistamatiou T, Stavropoulos-Giokas C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J Stem Cells. 2020 Aug 26;12(8):731-751. doi: 10.4252/wjsc.v12.i8.731. PMID: 32952855; PMCID: PMC7477656.
Chau AS, Weber AG, Maria NI, Narain S, Liu A, Hajizadeh N, Malhotra P, Bloom O, Marder G, Kaplan B. The Longitudinal Immune Response to Coronavirus Disease 2019: Chasing the Cytokine Storm. Arthritis Rheumatol. 2021 Jan;73(1):23-35. doi: 10.1002/art.41526. Epub 2020 Dec 1. PMID: 32929876.
Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant 2010; 19: 667- 679 [PMID: 20525442 DOI: 10.3727/096368910X508762]
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233: 6425-6440 [PMID: 29319160 DOI: 10.1002/jcp.26429]
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018 Sep;233(9):6425-6440. doi: 10.1002/jcp.26429. Epub 2018 Mar 1. PMID: 29319160.
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017 Feb 21;10(1):53. doi: 10.1186/s13045-017-0423-1. PMID: 28222796; PMCID: PMC5320663.
Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol. 2008;181:329–42.
Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14(6):405–15.
Dai H, Wang Y, Lu X, Han W. Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. 2016;108(7):djv439.
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–4
Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, Han D, Hong W, Wei W, Tu J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021 Jul;139:111605. doi: 10.1016/j.biopha.2021.111605. Epub 2021 Apr 23. PMID: 33901872.
Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 2020; 20: 269-270 [PMID: 32273594 DOI: 10.1038/s41577-020-0308-3]
Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846-848 [PMID: 32125452 DOI: 10.1007/s00134-020-05991-x]
Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020; 117: 10970-10975 [PMID: 32350134 DOI: 10.1073/pnas.2005615117]
Levine BL, Kessler DA, Rappel WJ. Gene Transfer in Humans Using a Conditionally Replicating Lentiviral Vector. Proc Natl Acad Sci (2006) 103:17372–7. doi: 10.1073/pnas.0608138103
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, et al. CAR Exosomes Derived From Effector CAR-T Cells Have Potent Antitumour Effects and Low Toxicity. Nat Commun (2019) 10:1–12. doi: 10.1038/s41467-019-12321-3
