
Our Project
Our project comprises two aims:
1. Design a novel synthetic anti-viral CAR-macrophage with immune regulatory capacity.
2. Demonstrate its feasibility through both in vitro experiments and mathematical modeling.
COVID-19 is a viral infection caused by SARS-CoV-2 which has affected the whole world. In the early stage of infection compared to common viral influenza, the symptoms are mild or don’t even manifest(Blanco-Melo et al. 2020). It is partly due to the delayed IFN-I release caused by viruses, which leads to inactivation of macrophage and postponed immune cells recruitment.(Chau et al. 2021; Wang et al. 2021).

Notably, the impaired immune responses lead to incomplete viral clearance. As the viruses replicate silently in the host, the disease develops into the late stage. An aberrant immune response occurs with increased proinflammatory cytokine release, finally resulting in cytokine release syndrome (CRS)(Chau et al. 2021).

Based on these, it is important to eradicate the viruses as early as possible by redirecting to the spike (S) protein and facilitating the immune response in the early course. It may lower the risk of developing into severe cases. Once entering the late stage of disease, it is critical to combat against viruses as well as to suppress the proinflammatory cytokine release, which avoid snowballing of immune response.

Toggle macrophage is a chimeric antigen receptor (CAR)-macrophage engineered with IL-6 sensor. We chose macrophages (M0) as the chassis because:
1.Macrophages participate in the innate immune response and play a critical role in the body’s defense against viral infections. They are a powerful activator for triggering adaptive immune responses and killing viruses(Meidaninikjeh et al. 2021).
2.Naturally, they can transform into M1 type and M2 type, which is pro-inflammatory and anti-inflammatory, respectively. This characteristic of macrophages corresponds to our ideal therapeutics that can eradicate the viruses and balance the immune response.
3.Delayed IFN-I release is associated with inactivation of macrophages, which later causes delayed viral clearance and increased proinflammatory cytokine release.
4.There is no reported evidence for the presence of infectious viruses or toxic products in THP-1 cells, making this cell line relatively easy and safe to use(Chanput, Mes, and Wichers 2014).
5.Recently, researchers have constructed CAR-macrophages, which exert their efficacy over killing the cancer cells. CAR-macrophages were endowed with the enhanced recognizing ability and phagocytosis. Therefore, we considered that CAR-macrophages would be a strong tool to treat SARS-CoV-2 infection.

We aimed to eradicate the viruses and facilitate the immune response in the early course, while combating against viruses as well as suppressing the proinflammatory cytokine release in the late course. However, clinically, it is difficult to precisely determine the transition point in the course of COVID-19, making it hard to inject two types of cells in sequence. Therefore, we decided to design a smart anti-viral macrophage that can automatically adjust its state of function in vivo by sensing the changes within the microenvironment.

Toggle Macrophage relies on a smart system consisting of five modules: IL-6 sensor 1, IL-6 sensor 2, CARγ, CARMERTK, and a kill switch. When IL-6 concentration is low within the microenvironment, the IL-6 sensor 1 and 2 are not activated. The expression of CARγ is activated. Toggle Macrophage thus acts as the CARγ-macrophage, killing the viruses and promoting the immune response. When IL-6 concentration is high within the microenvironment, the IL-6 sensor 1 and 2 are triggered. The expression of CARγ is inhibited and the expression of CARMERTK is activated. Toggle Macrophage thus acts as the CARMERTK-macrophage, killing the viruses and repressing the immune response. Consequently, Toggle Macrophage prevents the COVID-19 patients from deteriorating and ameliorates the condition of severe cases. Please visit Design Page to learn more details about the rationale of Toggle Macrophage.

Artificial macrophages:We engineer Toggle Macrophage with CAR and cytokine sensor, creating a novel artificial macrophage against SARS-CoV-2.
Phenotypic transformation: Toggle Macrophage can transform its phenotype according to the IL-6 levels in the microenvironment.
Sensing IL-6: With the IL-6 sensor on the surface of Toggle Macrophage, it can sense the IL-6 concentration within the microenvironment.
“Smart”: By sensing the IL-6 levels, Toggle Macrophage can automatically switch its phenotype once the hyperinflammation occurs.
Model: We use mathematical modeling to study the problems that can’t be solved in in vitro/vivo experiment. For example, the clinical transition of various individuals can be postulated by modeling, providing a guidance for therapy design. We also employ the modeling to study the impact of CAR-macrophages on the IL-6 levels.
Our Inspiration
Background: Early in February 2020, COVID-19 spread the whole world. Till now, there have been more than 200 million confirmed cases and 4 million deaths according to WHO. Notably, 5%–15% of patients progress from mild disease to severe viral pneumonia and hypoxemic respiratory failure, followed by a hyperinflammatory response (Chau et al. 2021). According to Professor Li, cytokine storm is a primary cause of deterioration and death in COVID-19 patients. (See more in Human Practice) As medical students, we endeavor to do our part in fighting against COVID-19. Therefore, we determine to initially focus on providing a specific therapy for patients with severe COVID-19.
Current Treatments: Professor Li, a member of the support team, said in our interview that current treatments against COVID-19-induced inflammation are mainly non-specific and supportive therapy, such as ECMO and ventilator.
(See more details in Human Practice) Recently, researchers have developed several antibodies targeting cytokines. Data showed that the rational use of the tocilizumab (IL-6 inhibitor) in severe and critical COVID-19 patients can prevent the development of irreversible lung injury and death of the patients (Kulanthaivel et al. 2021; Kaye and Siegel 2020). Several clinical trials testing tocilizumab are ongoing (NCT04445272, NCT04317092). Myriads of resources and investment have been put into the development of various vaccines against SARS-CoV-2, such as mRNA vaccines, adenovirus vaccines, and inactivated vaccines. It becomes clear that vaccines have protected people worldwide from COVID-19 (Baden et al. 2021; Voysey et al. 2021; Zhang et al. 2021). However, the continuous mutation of SARS-CoV-2 poses a potential threat to the efficacy of vaccines. It was reported that hyperimmune globulins from convalescent plasma do benefits in treating viral infection of coronaviruses. However, animal models indicated that hyperimmune globulins led to a harmful, enhanced inflammatory response in a process termed antibody-dependent enhancement (ADE) (Nguyen et al. 2020). Small molecule drugs are another strategy to treat COVID-19, such as chloroquine and hydroxychloroquine, antiretrovirals, Ribavirin, and anti-viral drugs. It was reviewed that no strong evidence suggested a clear benefit of (hydroxy)chloroquine in treating COVID-19 patients (Wiersinga et al. 2020). Similarly, no clear evidence confirmed the efficacy of antiretrovirals like Ribavirin and lopinavir/ritonavir (Sanders et al. 2020). A study showed that remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with COVID-19 and had evidence of relieving respiratory tract infection (Beigel et al. 2020). However, a clinical trial reported that remdesivir didn’t have statistically significant clinical benefits in patients with severe COVID-19 (Wang et al. 2020). Conclusively, despite the variety of therapies, methods for severe COVID-19 seem to be limited.

In the early stage of SARS-CoV-2 infection, delayed release of cytokines and chemokines occurs in respiratory epithelial cells, dendritic cells (DCs), and macrophages. Later, the cells secrete low levels of the antiviral factors interferons (IFNs) and high levels of proinflammatory cytokines and chemokines. On the one hand, many inflammatory cells are attracted, resulting in excessive infiltration of the inflammatory cells into lung tissue and thus causing lung injury (Ye, Wang, and Mao 2020; Chau et al. 2021). On the other hand, the interaction of Fas–Fas ligand (FasL) and TRAIL–death receptor 5 (DR5) during the infiltration causes the apoptosis of airway and alveolar epithelial cells, leading to hypoxia in the body (Ye, Wang, and Mao 2020). Thus, inflammatory mediators play a key role in the pathogenesis of acute respiratory distress syndrome (ARDS). The cytokine storm is also a key factor in determining the clinical course of extrapulmonary multiple-organ failure (Chau et al. 2021; Ye, Wang, and Mao 2020). In conclusion, cytokine storm is closely associated with severe COVID-19 and is thus deemed as a promising target of therapeutic strategy.
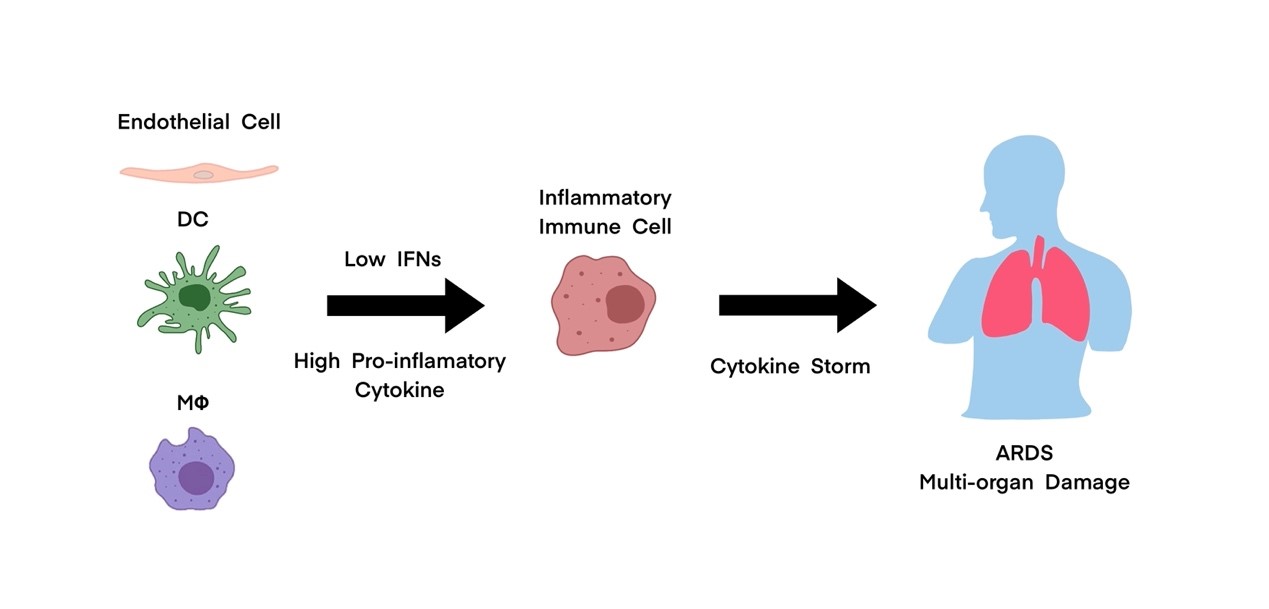
Macrophages are ubiquitous throughout the body. Alveolar macrophages (AMs) are likely the first immune cells to encounter SARS-CoV-2 during an infection. A study found that AMs are incapable of sensing SARS-CoV-2 and producing an IFN response (Dalskov et al. 2020), which explained the asymptomatic phase during COVID-19. The delayed IFNs release led to incomplete viral clearance, later resulting in high virus load and increased proinflammatory cytokines (Chau et al. 2021). Then, macrophages are activated by IFNs and thus producing more monocyte chemo-attractants (such as CCL2, CCL7, and CCL12), resulting in abundant accumulation of macrophages. Macrophages also release proinflammatory cytokines to recruit other immune cells and cause hyperinflammation (Ye, Wang, and Mao 2020). Their dysregulated functions lead to acute lung injury, ARDS and multiple organ damages (Meidaninikjeh et al. 2021; Kosyreva et al. 2021). As can be seen, macrophages are both friends and foes in COVID-19. Our project is to create a novel macrophage as our loyal backup against COVID-19.
Our Project Goals
We determined to create a smart therapy based on macrophage against COVID-19. More importantly, we hoped to provide a new strategy for treating cytokine storm-related diseases. To confirm the feasibility of our project, we have done the following work:
1.Conduct literature review on the role of macrophage in cytokine storm occurred in COVID-19.
2.Identify the feasible genetic parts for enhancing phagocytosis and controlling the phenotypic switch of Toggle Macrophage.
3.Conduct in vitro experiments to select ideal CAR for Toggle Macrophage and confirm the efficacy of CAR in order to determine the effectiveness of Toggle Macrophage to phagocytose the viruses.
4.Establish mathematical model to determine the feasibility of Toggle Macrophage, such as the impact of Toggle Macrophage on the IL-6 levels. Also, we obtained guidance from modeling on the injection volume of Toggle Macrophage, making it suitable for different individuals.
5.Consult medical stuffs who have experiences in treating COVID-19 for clinical advises of therapeutic methods and clinical application of Toggle Macrophage. This gave us a hint for further experiments and modeling.
6.Consult experts in immunology for inspiration and feedbacks on our design of Toggle Macrophage so as to make it innovative, safe, effective, and convenient enough.
7.Develop medical corporation and refer to stakeholders about the implementation and regulations of Toggle Macrophage if conducted in the future.
How COVID-19 Impacted Our Project:
COVID-19 is closely related to our project and affects our project in many aspects. Here, we would like to talk mainly about its negative impact on us, such as not being able to hold large off-line meetings, which is a hurdle for our human practice; and not being able to participate in conferences in other cities, which has hindered the collaboration and partnership of our project to some extent. Thanks to the strong prevention and control of our country, we can return to school in time to carry out the experiments smoothly. In short, COVID-19 has been influencing our project, while we are reciprocally influencing it as well.
Reference
Baden, L. R., H. M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S. A. Spector, N. Rouphael, C. B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B. S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. P. Deng, H. H. Zhou, S. Han, M. Ivarsson, J. Miller, T. Zaks, and Cove Study Grp. 2021. 'Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine', New England Journal of Medicine, 384: 403-16.
Beigel, J. H., K. M. Tomashek, L. E. Dodd, A. K. Mehta, B. S. Zingman, A. C. Kalil, E. Hohmann, H. Y. Chu, A. Luetkemeyer, S. Kline, D. L. de Castilla, R. W. Finberg, K. Dierberg, V. Tapson, L. Hsieh, T. F. Patterson, R. Paredes, D. A. Sweeney, W. R. Short, G. Touloumi, D. C. Lye, N. Ohmagari, M. D. Oh, G. M. Ruiz-Palacios, T. Benfield, G. Fatkenheuer, M. G. Kortepeter, R. L. Atmar, C. B. Creech, J. Lundgren, A. G. Babiker, S. Pett, J. D. Neaton, T. H. Burgess, T. Bonnett, M. Green, M. Makowski, A. Osinusi, S. Nayak, H. C. Lane, and Actt- Study Grp. 2020. 'Remdesivir for the Treatment of Covid-19-Final Report', New England Journal of Medicine, 383: 1813-26.
Blanco-Melo, Daniel, Benjamin E. Nilsson-Payant, Wen-Chun Liu, Skyler Uhl, Daisy Hoagland, Rasmus Møller, Tristan X. Jordan, Kohei Oishi, Maryline Panis, David Sachs, Taia T. Wang, Robert E. Schwartz, Jean K. Lim, Randy A. Albrecht, and Benjamin R. tenOever. 2020. 'Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19', Cell, 181: 1036-45.e9.
Chanput, W., J. J. Mes, and H. J. Wichers. 2014. 'THP-1 cell line: An in vitro cell model for immune modulation approach', International Immunopharmacology, 23: 37-45.
Chau, A. S., A. G. Weber, N. I. Maria, S. Narain, A. Liu, N. Hajizadeh, P. Malhotra, O. Bloom, G. Marder, and B. Kaplan. 2021. 'The Longitudinal Immune Response to Coronavirus Disease 2019: Chasing the Cytokine Storm', Arthritis Rheumatol, 73: 23-35.
Dalskov, L., M. Mohlenberg, J. Thyrsted, J. Blay-Cadanet, E. T. Poulsen, B. H. Folkersen, S. H. Skaarup, D. Olagnier, L. Reinert, J. J. Enghild, H. J. Hoffmann, C. K. Holm, and R. Hartmann. 2020. 'SARS-CoV-2 evades immune detection in alveolar macrophages', Embo Reports, 21.
Kaye, A. G., and R. Siegel. 2020. 'The efficacy of IL-6 inhibitor Tocilizumab in reducing severe COVID-19 mortality: a systematic review', Peerj, 8.
Kosyreva, A., D. Dzhalilova, A. Lokhonina, P. Vishnyakova, and T. Fatkhudinov. 2021. 'The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome', Frontiers in Immunology, 12.
Kulanthaivel, S., V. B. Kaliberdenko, K. Balasundaram, M. V. Shterenshis, E. Scarpellini, and L. Abenavoli. 2021. 'Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review', Reviews on Recent Clinical Trials, 16: 138-45.
Meidaninikjeh, S., N. Sabouni, H. Z. Marzouni, S. Bengar, A. Khalili, and R. Jafari. 2021. 'Monocytes and macrophages in COVID-19: Friends and foes', Life Sciences, 269.
Nguyen, A. A., S. B. Habiballah, C. D. Platt, R. S. Geha, J. S. Chou, and D. R. McDonald. 2020. 'Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution!', Clinical Immunology, 216.
Sanders, J. M., M. L. Monogue, T. Z. Jodlowski, and J. B. Cutrell. 2020. 'Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19) A Review', Jama-Journal of the American Medical Association, 323: 1824-36.
Voysey, M., S. A. C. Clemens, S. A. Madhi, L. Y. Weckx, P. M. Folegatti, P. K. Aley, B. Angus, V. L. Baillie, S. L. Barnabas, Q. E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A. M. Collins, R. Colin-Jones, C. L. Cutland, T. C. Darton, K. Dheda, C. J. A. Duncan, K. R. W. Emary, K. J. Ewer, L. Fairlie, S. N. Faust, S. Feng, D. M. Ferreira, A. Finn, A. L. Goodman, C. M. Green, C. A. Green, P. T. Heath, C. Hill, H. Hill, I. Hirsch, S. H. C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C. C. D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A. M. Lawrie, A. Lelliott, V. Libri, P. J. Lillie, R. Mallory, A. V. A. Mendes, E. P. Milan, A. M. Minassian, A. McGregor, H. Morrison, Y. F. Mujadidi, A. Nana, P. J. O'Reilly, S. D. Padayachee, A. Pittella, E. Plested, K. M. Pollock, M. N. Ramasamy, S. Rhead, A. V. Schwarzbold, N. Singh, A. Smith, R. Song, M. D. Snape, E. Sprinz, R. K. Sutherland, R. Tarrant, E. C. Thomson, M. E. Torok, M. Toshner, D. P. J. Turner, J. Vekemans, T. L. Villafana, M. E. E. Watson, C. J. Williams, A. D. Douglas, A. V. S. Hill, T. Lambe, S. C. Gilbert, A. J. Pollard, and Covid Vaccine Trial Grp Oxford. 2021. 'Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK', Lancet, 397: 99-111.
Wang, Y. M., D. Y. Zhang, G. H. Du, R. H. Du, J. P. Zhao, Y. Jin, S. Z. Fu, L. Gao, Z. S. Cheng, Q. F. Lu, Y. Hu, G. W. Luo, K. Wang, Y. Lu, H. D. Li, S. Z. Wang, S. N. Ruan, C. Q. Yang, C. L. Mei, Y. Wang, D. Ding, F. Wu, X. Tang, X. Z. Ye, Y. C. Ye, B. Liu, J. Yang, W. Yin, A. L. Wang, G. H. Fan, F. Zhou, Z. B. Liu, X. Y. Gu, J. Y. Xu, L. H. Shang, Y. Zhang, L. J. Cao, T. T. Guo, Y. Wan, H. Qin, Y. S. Jiang, T. Jaki, F. G. Hayden, P. W. Horby, B. Cao, and C. Wang. 2020. 'Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial', Lancet, 395: 1569-78.
Wang, Zhihui, Ming Zhou, Zhenfang Fu, and Ling Zhao. 2021. 'The Pathogenic Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Possible Mechanisms for Immune Evasion?', Frontiers in Immunology, 12.
Wiersinga, W. J., A. Rhodes, A. C. Cheng, S. J. Peacock, and H. C. Prescott. 2020. 'Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review', Jama-Journal of the American Medical Association, 324: 782-93.
Ye, Q., B. L. Wang, and J. H. Mao. 2020. 'The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19', Journal of Infection, 80: 607-13.
Zhang, Y. J., G. Zeng, H. X. Pan, C. G. Li, Y. L. Hu, K. Chu, W. X. Han, Z. Chen, R. Tang, W. D. Yin, X. Chen, Y. S. Hu, X. Y. Liu, C. B. Jiang, J. X. Li, M. N. Yang, Y. Song, X. X. Wang, Q. Gao, and F. C. Zhu. 2021. 'Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial', Lancet Infectious Diseases, 21: 181-92.
