Introduction
To realize hair dyeing & perming and decoloring & straightening as designed, we use E.coli to construct all the needed plasmid and transfect them into yeast through electroporation to express target enzymes and peptides. To confirm the validity of our perming and dyeing theory and eventually, the whole project, real hair is used in lab trails, which aim to estimate its color fastness, mechanical property, etc.
Plasmid construction and amplification
The transformation of plasmid with AOX1 as promoter
First of all, we need to amplificated all the commercially synthesized plasmid to acquire enough amount for further study. After transformation, colony PCR is applied for confirmation. Then we go for plasmid extraction.

Figure 1: Colony PCR confirmation of successful E.coli transfection
Bright bands of identical sizes from colony PCR result demonstrates that target plasmid had successfully transformed into E.coli
The construction of plasmid with Panb1 or Pynr071c as promoter
AOX1 promoter is the strongest eukaryotic promoter currently known in yeast expression system. So we choose AOX1 as the primary promoter when we synthesized all these plasmid for the sake of more convenient expression. But noticing that methanol is hazardous, flammable, combustible and therefore, inappropriate to have direct contact with the hair, we need to substrate AOX1 for constitutive promoter Panb1 and xylose induced promoter Pynr071C to realize the projected regulation function as designed. Double-enzyme cleavage and rejointing is used to achieve this. We amplify the target gene located in the primarily synthesized plasmid without AOX1 promoter, and digest the acquired fragments and two kinds of plasmid, containing promoter only, with EcoR I and BamH I, then transform them into E.coli after linking the product together. Through this, we successfully substrate the primary AOX1 for Panb1 and Pynr071C.

Figure 7: Plasmid construction and colony PCR results of reconstructed plasmid with Panb1 and Pynr071C promoter
All the bands are identical to the theoretical lengths, which could demonstrate that these plasmid are correctly constructed and successfully transformed into E.coli, confirmed by sequencing.
The construction of plasmid without signal peptide
During the experiment, we find that not all the protein we want could be secreted extracellularly. Target protein is only detected in the yeast but not in the culture. while no signal appears in the supernatant without them, which indicates that not all of our signal peptides works. We reckon that as the α-factor on the plasmid we used is only a common basic signal peptide, which has some degree of universality but couldn't fit all the protein due to unseen matters like space structure of the protein, our target protein couldn't be induced to secret into the extracellular space by α-factor, or the low expression level coupled with degradation from protease existing in the extracellular environment could do the same. So, to get the rest of our enzymes which don't want to be outside of the cell with α-factor, and whether or not we could raise the expression level, we design new primers for PCR, which eliminate the signal peptide part off the whole target genes, and reconstruct the plasmid without the α-factor to verify if they prefer to be expressed inside of the cell.

Figure 11: Plasmid construction and colony PCR results of plasmid without signal peptides transformed E.coli
All the bands are identical to the theoretical lengths, which could demonstrate that these plasmid are correctly constructed and successfully transformed into E.coli, confirmed by sequencing.
Exploring the construction of plasmid for pigment synthesis
After all the expression product is detected, to explore the molecular mechanism laid behind and to save more experience for future teams, we tried to construct the complete whole pathways. Using seamless cloning, tentative experiments are performed by new primer for homologous recombination.
Unfortunately, no plasmid with multiple target genes are constructed. Good news is double-target-gene plasmid obtained. It seems that the identical promoters and terminators lead to the much shorter product of seamless cloning than theoretical, which means the enzymes used to join the designed homologous arms by primers falsely take some of the shared promoters and terminators as substrates as well. Although FMO dimers were successfully obtained, other plasmids were always shorter than expected .Then we give double-enzyme digestion and reconnection a try but limited to the restriction sites existed in our target genes and more fragments less odds and, well, time, no complete pathway is constructed. What we do have now is the precious experimental experience for all of our team members, and useful information for future teams.
Electroporation and expression of yeast
To verify the expression of our genes and to acquire corresponding enzymes, we choose Pichia Pastoris GS115 as chassis and using electroporation to blend our plasmids into them. Large amount of target gene containing plasmid is extracted, then we digest them with Bgl II or Sal I to get linear plasmid, which could be integrated into yeast genome to avoid getting lost while being frozen. Concentration is also applied to achieve higher copy number and higher expression level.
After electroporation of plasmid with AOX1 as promoter and those without signal peptide, we acquire identical bands through Nickel-affinity chromatography column and SDS-PAGE.
For some of our enzymes don't have standard protocol to estimate their activity at present, we add substrates into culturing medium accordingly to find out whether there exists active target enzymes and do get our indigo and lycopene synthesized.

Figure 16: Medium for expression with substrates From left to right: GS115 medium with indole and FPP as control; Panb1-FMO-AOX1 Terminator medium with indole; mixture of Panb1-crtE-AOX1 Terminator, Panb1-crtB-AOX1 Terminator, Panb1-crtI-AOX1 Terminator medium with FPP
From left to right: GS115 medium with indole and FPP as control; Panb1-FMO-AOX1 Terminator medium with indole; mixture of Panb1-crtE-AOX1 Terminator, Panb1-crtB-AOX1 Terminator, Panb1-crtI-AOX1 Terminator medium with FPP
Also, In order to verify whether the catalytic effect of FMO dimer is better than FMO. We electrotransformed the FMO dimer into yeast and tried to detect its expression and compare it with FMO.
The color of the medium and the cell were significantly darker than FMO under the same culture conditions. This shows that the catalytic activity of FMO dimer is stronger than that of FMO.

Figure 27: FMO dimer medium with indole after centrifugation.It is obvious that the culture medium and bacteria are dark blue.
Determination of enzyme avtivity
After the target strips appeared on SDS-PAGE, for the enzymes with standard enzyme activity assay such as Laccase and LOX2, we measured their enzyme activity according to the standard measurement methods to detect whether our enzymes are catalytically active. Laccase and LOX2 show high activity after adjusting the relevant conditions such as pH, temperature and ion concentration

Figure 31: Enzyme activity determination of Laccase

Figure 32: Enzyme activity determination of LOX2
Verification of promoters
As we had successfully used Panb1 as promoter to express our protein, which can confirm that the Panb1 promoter can work smoothly. So to verify whether our Pynr071c promoter could initiate the expression of downstream genes, we linked GFP it, then transfect it into yeast and test the exist of fluorescence.

Figure 36: Fluorescence result of Pynr071c-GFP-AOX1 Terminator
The Experiment Standard
We measured the standard curves of three pigments before using them for hair dyeing experiment. We also found that the amount of melanin contained in hair can have a significant effect on hair dyeing outcomes. Therefore, we define different colors of hair based on bleaching.
The Best Condition of hair dye
We have gained the best dye conditions of three kinds of hair dye(indigo, curcumin and lycopene) at a certain concentration. Under optimal conditions, we dyed 4-9 degrees of hair to get a series of dyeing discs. And we found that as for the three colors selected for the experiment, bleach the hair to 8 degrees could achieve a bright coloring effect.
Hair stress experiment
We used a micro scale experimental instrument to quantitatively evaluate the changes in mechanical properties of hair after hair dyeing. The results show that Indigo and lycopene as dyes have no obvious damage to hair. Curcumin has a repairing effect on hair.

Figure 51: Tensile Stress Strain Curves
Compound hair dye paste
After finishing the solution experiment, we try to mix the natural pigment into a dye that can be applied directly to the hair. At present, lycopene dye and curcumin dye with NO.1 cream matrix as carrier are obtained, and natural essence is added to improve the odor of dye paste. Indigo is an oxidizing dye with special properties, so we designed a timely fermenter. In this way, we can use our product right now when indigo is produced and reduced to indigo white.

Figure 53: The expected product
Color fastness test
Color fastness is an important aspect to measure the effect of dye, so we design a set of elution scheme and test the color fastness of three kinds of natural pigment dye products and the same color traditional dye paste. The results showed that the color fastness of the natural pigment dyes was better than that of the traditional dyes.

Figure 56: The comparison of biological dying with traditional dying
Toxicological experiment of broad bean root tip
In order to test the safety of our dye paste, we carried out a toxicology experiment of broad bean root tip. The rate of micronucleus in root tip cells on broad bean was measured. The higher the micronucleus rate was, the higher the teratogenic rate was. The results showed that the teratogenicity of our product was significantly lower than that of the two traditional hair dyes on the market, indicating that our product was less toxic.

Figure 57: Micronucleus Frequency
Color stability testing
We would expose our hair dyed with our dye paste and two chemical dyes (red and blue) to constant bright light, take pictures before and after lighting, and calculate the degree of discoloration. The result shows that the light stability of our dye paste is slightly less than that of the chemical dye paste, but the difference is invisible to the naked eye in the actual application scene, so the color of our dye paste can exist stably in the natural light, it's still very practical.

Figure 60: Light exposure's influence on dying
Color matching experiment and Sumu experiment
By adding color aids, mixing two dyes or secondary coloring, we get more hair colors. Now, we have different brightness of the rainbow seven colors and gray, red, brown, tawny and other colors. At the same time, we also used hematoxylin as a dye to explore a series of dyeing. Hematoxylin itself can be brown, add alum can be red, add alkali can be purple. Hematoxylin 's synthetic biology production will be a future extension project.

Figure 61: Olympic rings and Cartoon kid

Figure 62: the coloring effect of hematoxylin under various conditions
Experiment of short peptide perm
Short peptides perming hair experiment

Figure 63: comparison of short peptides and water
We used 0.1% and 0.01% short peptides for perm experiment, and set chemical perm and up water perm as control group. The perming effect of short peptide is more obvious than that of ultra-pure water, which shows that in alkaline and 50° conditions, the hair cuticle is opened and the longer react time it takes, the better perming result we get. And after a series experiment, we found that hair dealt with SDS can have more obvious curl effect.

Figure 64: comparison of short peptides with different mixture with other chemicals
Perming hair stress experiment-9
We used different kinds of perms for hairs with 2 groups of different hair, and the hair strains will be damaged after perming. So we designed and conducted stress experiments to test the mechanics of the hair after perming. The figure below shows the experiment data, by which we can know that perming by short peptides will have very little damage to the hair, and short peptide perm damage is less than chemical perm. Moreover, the results of the damaged hair groups showed that the damage of short peptide perm is much smaller than that of chemical perm. Hence, we speculated that perming procedure would cause some damage to hair, but the use of short peptide perm could be used to repair the damaged hair.
Healthy hair stress test results (Horizontal: the deformation length; Ordinate: pull force
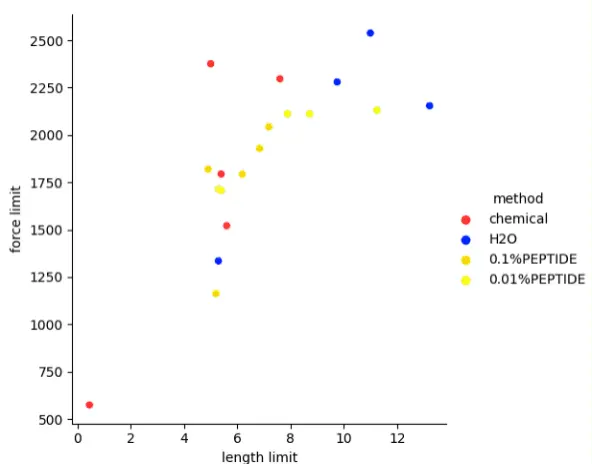
Figure 65: Healthy hair stress test results (Horizontal: the deformation length; Ordinate: pull force)
It is shown that the hair of the short peptide perm group changes in shape under constantly increasing pull force, and most hair strains can withstand more pull than hair permed by chemical perms. This suggests that short peptide perms do less damage to hair performance than chemical perms. According to the experimental results, we judge that the concentration of short peptides has little effect on the results.
Damaged hair stress test results (Horizontal: the maximum length; Ordinate: pull force)

Figure 66: Damaged hair stress test results (Horizontal: the maximum length; Ordinate: pull force
This set of data comes from a pulling test of damaged hair. It is shown in this figure that both sets of hairs that use short peptides for perm can withstand more pull than the hairs after chemical perm. And there isn't much difference between the results of short peptide group and that of the water group. We speculate that short peptides can by some means repair the damaged hair, which also portrays the excellent performance of short peptide perm that it can protect the hair and maintain the hair quality.
Microscopic effects of PepACS on hair
We used scanning electron microscopy to look at healthy hair, bleached hair and bleached hair treated with the PepACS. The results showed that compared with normal hair, the surface of bleached hair was obviously damaged, but no obvious damage was observed after treatment with PepACS, indicating that PepACS may have a certain repair effect, which can be further studied in the future.

Wow! That’s eye-opening! I guess the old saying that “Rome was not build in a day” does make sense. All those results require accumulation, which is recorded in the notebook.


























































