
As we successfully constructed a synthetic collagen plasmid (BBa_K3956014) with three fragments and verified its presence on a gel, we simultaneously worked on the proof of concept of our project using mammalian collagen. Our final goals are to create quality biomedical gels and threads for use in tissue engineering and further biofabrication research.
Hardware:
The spinning machine design we used was created by Great Bay’s iGEM team of 2019. Their purpose for using the machine was to produce synthetically made spider silk, similar to our biosynthetic collagen. Necessary modifications to the design were made to account for the difference in protein, and the collagen solution was suspended in 8.8mM acetic acid and threaded through 70% isopropanol, all of which are proven methods for collagen threading by previous literature (Cavallaro et. al, 1994).
Functionally, the machine works properly and has the necessary controls to run at lower and constant rpm. Because Great Bay’s implementation of the design was successful in spinning silk threads, we rationalized that using the same design on similar material (collagen) would also produce thread.

The machine is functional and has the required controls to run at a lower and constant rpm. Because Great Bay's implementation of the design was successful in spinning silk threads, we reasoned that we could utilize the same design on a similar material (collagen) and produce thread.
Read more at our Hardware Page
Gelification:
Collagen gels and matrices are already used in skin graft procedures with great success. In a study by Chattopadhyay et al., 2014, a collagen gel was used for wound healing and tissue engineering and was demonstrated to be nontoxic and a high tensile strength. Furthermore, Strong evidence from recent literature supports vascular applications of collagen gel. In a 2000 study by Song et al. demonstrated blood vessel formation in vitro through a collagen matrix environment. Additionally, collagen gels are widely used in tissue culture research as an abundant anchoring matrix protein. As a result, to demonstrate the feasibility of transforming collagen protein into medically applicable bio-material, we coagulated collagen into collagen gels.
We went through many iterations of creating the gel (Engineering), but our most successful iteration used a coagulation buffer that consisted of a PEG based buffer. This allowed for the collagen to coagulate, and the addition of glutaraldehyde, a common crosslinking reagent, was added to stiffen and preserve the structural integrity. Aftwards, we removed all supernatant in order for the gel to settle into a 1 cm circular disk.
Although the collagen source used for these experiments was mammalian pig collagen instead of bacterial Scl2 collagen, previous literature has confirmed that structural similarity would provide comparable results (Peng et al., 2012; Xu et al., 2002). This is because the triple helical structures that give both collagens their characteristic strength are quite similar, with Gly-Xaa-Yaa repeats. Furthermore, our plasmid design incorporated parts that cleave the globular domain of synthetic bacterial collagen from the collagen-like domain, which speerates the triple helices from the N-terminus. This is important because it mimics the natural MMP collagenase cut site in human collagen, which further supports that the Scl2 collagen can be used for human biomedical applications.
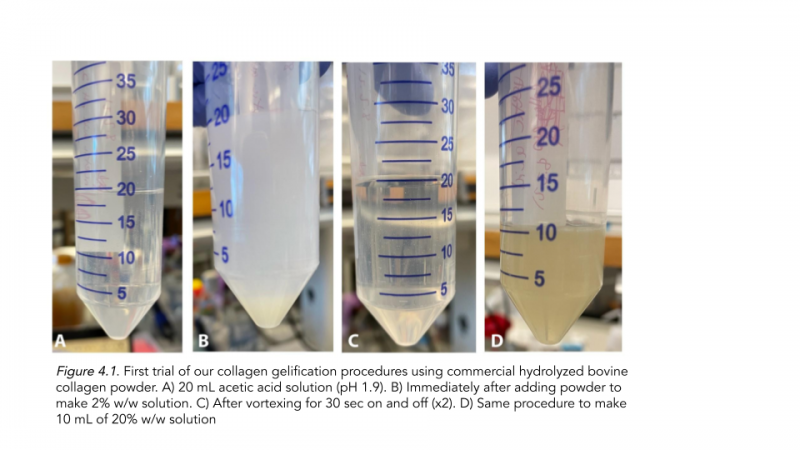
For our first trial of collagen gelification (Figure 4.1), we aimed to test out commercial hydrolyzed bovine collagen powder. After adding acetic acid to create a 2% solution, we discovered that our collagen sample did not gel, likely due to impurities in the powder. As a result, we decided to use porcine bovine collagen to further test gelification.

First we obtained local pig skin and removed all hair and connective tissue. We then proceeded to wash the pig skin in a sodium citrate buffer and added pepsin to the telopeptide rich collagen as done by Cliche et. al, 2003, which would remove telopeptides from the collagen-like domain. We ran both telopeptide-rich collagen and telopeptide-poor collagen cleaved with pepsin in an SDS-PAGE. Surprisingly, we found that the molecular weight of bacterial collageen closely matched that of telopeptide-poor mammalian collagen around 40 kDa (Figure 2.2). Thus we were able to conclude that the pepsin digest mimics the proteolysis of Scl2 collagen, which makes it a reliable source to use in our subsequent gelification procedures.


To begin testing the gellification after sourcing proper collagen we made an initial fiber formation buffer, which consisted of NaCl, sodium phosphate dibasic heptahydrate, and TES buffer. However, this did not demonstrate substantial coagulation. As a result, we decided to create a coagulation buffer that consisted of glutaraldehyde, PBS, and deionized water. The 80% w/w collagen solution showed instant coagulation after the supernatant was removed (Figure 4.3).
References
Cliche, J Amiot, C Avezard, C Gariepy, Extraction and characterization of collagen with or without telopeptides from chicken skin, Poultry Science, Volume 82, Issue 3, 2003, Pages 503-509, ISSN 0032-5791, https://doi.org/10.1093/ps/82.3.503.
Chattopadhyay, S., & Raines, R. T. (2014). Collagen‐based biomaterials for wound healing. Biopolymers, 101(8), 821–833.https://doi.org/10.1002/bip.22486
Cavallaro, J. F., Kemp, P. D., & Kraus, K. H. (1994). Collagen fabrics as biomaterials. Biotechnology and Bioengineering, 43(8), 781–791. doi:10.1002/bit.260430813
Peng, Y.Y., Howell, L., Stoichevska, V. et al. Towards scalable production of a collagen-like protein from Streptococcus pyogenes for biomedical applications. Microb Cell Fact 11, 146 (2012). https://doi.org/10.1186/1475-2859-11-146
Song, J., Rolfe, B. E., Hayward, I. P., Campbell, G. R., & Campbell, J. H. (2000). Effects of collagen gel configuration on behavior of vascular smooth muscle cells in vitro: Association with vascular morphogenesis. In Vitro Cellular & Developmental Biology - Animal. Retrieved October 19, 2021, from https://link.springer.com/article/10.1007/BF02577528.
Xu Y, Keene DR, Bujnicki JM, Höök M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002 Jul 26;277(30):27312-8. doi: 10.1074/jbc.M201163200. Epub 2002 Apr 25. PMID: 11976327.









