
Overview
This page focuses on the bioreactor that could be used to improve production and cost efficiency. Through experiments done by our lab division, data collected was used for the basis of the bioreactors model. Issues regarding the wet-spinning machines relating to our project are brought up, and solutions are provided.
Yeast Bioreactor
One of our main goals for implementing Collagene is mass production. Mass producing collagen will allow for the biomaterial to be used in industry application through the form of felt, gel, or thread. In order for large amounts of Collagene collagen to be produced, large amounts of yeast are necessary as well. After researching the most optimal conditions of yeast growth and cultivation, we designed a cheap and efficient yeast bioreactor.
The Problem
Bioreactors straddle a fine line between simplicity and efficiency; oftentimes, designs will only be able to capture one. True mass production relies not only on efficient production, but also cost efficiency as well. Given our group’s funding limitations and industry goals, it is necessary to have a cheap yet effective design.
The Solution
We aimed to make a solution for the problem by designing a simple and efficient yeast bioreactor. The design is a single chamber that can be fitted with pressure gauges, an aerator, and an agitator, as well as measuring devices. This versatility allows it to function effectively with bare bone requirements, a great save on costs of production.

Another testament to its versatility is the bioreactor’s scaling. The single chamber design can be scaled to different sizes depending on the purpose of use; the only change necessary would be the motor and measuring equipment.

The efficiency of the system revolves around its specifications and reagents. First, the motors spin at 350 rpm. The quad propeller system covers the full length of the container, agitating as much yeast as possible inside. Its pointed edge, yet flat design, is specified to move freely as well as pushing the maximum amount of yeast media. Higher rpm is also shown to correlate with better yeast growth, as spinning allows for better gas exchange. Larger containers coincide with the motor specifications as larger containers create more surface area for gas exchange.

The optimal pH for yeast growth is 6 pH. Inside our bioreactor system, this is maintained via NaOH balancing, scaled to the selected size of the bioreactor. For temperature control, the only specification necessary is a constant 30 C temperature that can be accomplished by a heat source on the bottom of the container.

Air flow is necessary for the system as well to initiate gas exchange and create a uniform distribution of current that mixes the yeast medium. Pumping oxygen into the container is also necessary for the yeast to survive. The holes at the bottom of the container allow oxygen to pass through in equal amounts and spread evenly. Oxygen can be inserted via the protruding oval shaped tube at the bottom of the machine. As the yeast produces CO2 from processing the oxygen, pH can fall, so necessary measurements and responses are needed if the user desires maximum efficiency.

Pressure within the tank also contributes to more efficient growth rates. Below 10 psi of pressure can be measured with a pressure gauge and sealed with the lid of the container and input tubes. Of course, the machine is versatile, so it can function properly without optimum pressure and even without a lid.
The two tubes at the top of the machine are for securely inputting yeast growth media and water/NaOH. The input tube design allows for pressure management to be possible.
Finally, the bottom of the bioreactor has an effluent tube for automatic release of product if desired.

Results
The finished design is capable of being produced in 3D print and conventional material. The parts necessary range from a small number of cheap materials to possibly a massive amount of material depending on how large a bioreactor is needed. The single chamber design, however, allows for a large scaled version of the bioreactor to be more cost efficient. For a 20 inch wide bioreactor, the expected yeast density over time is represented below.
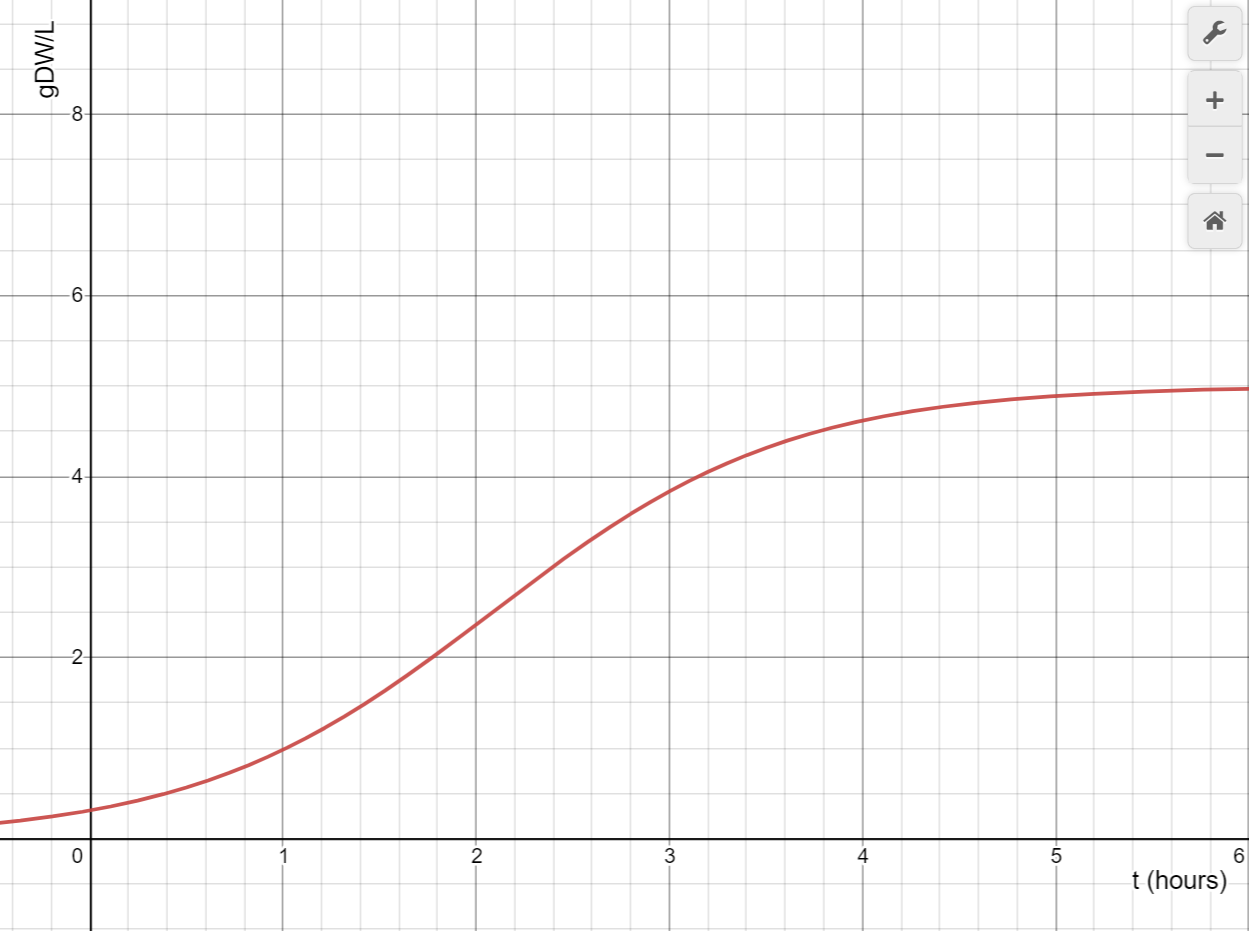

Our design was made in onshape CAD software. The schematics for the entire machine, except for measuring devices and the motor, are available as a downloadable file. Measurements can be altered after uploading the parts and can also be 3D printed.
Wet-Spinning Machine
One of our implementation goals for Collagene is collagen thread that can be used in medicine for crafting blood vessels and meshes. After researching effective ways of producing this, we decided on a wet-spinning machine.

The Problem
Too many machines made for the process of spinning fibres are complicated beyond measure, often involving numerous coagulation baths, strict temperature control, and even air flow manipulation. Not to mention the cost of said machines, recreating the machinery necessary for thread spinning is near impossible for individuals and even labs.
The Solution
We aimed to solve the issue by making an easily replicable thread spinning device. Our first lead was GreatBay iGEM 2019’s wet-spinning machine. Their design of spinning machine successfully threaded synthetic spider silk into strings. Its size and cost are noticeably diminished compared to competing apparatuses.
However, we noticed that certain aspects such as the thread dyer and automated microsyringe were unnecessary for our purposes and for thread spinning in general. Thus, our rendition of their design cuts more of these expensive parts and uses modified reagents specified for collagen. Specifically, 8.8mM acetic acid and 70% isopropanol are used as baths —proven methods for collagen threading by previous literature (Cavallaro et al. 1994).

Seen in Figure 5 above is our prototype version of the GreatBay design. Essentially, the only parts required are a motor, a spool, a power source, a speed controller (if necessary), and a plastic tub with reagents. The overall price is near miniscule as most materials can be found in home environments.
Results
Our prototype version successfully operated, spinning the motor at designated speeds, slow enough that thread would be able to form. Unfortunately, we were unable to test the design with actual threading materials due to time and material constraints. However, we rationalize that because the design successfully spun synthetic threads as demonstrated by GreatBay, the same result can be expected with our synthetic material.
Recreation
As previously stated, our wet-spinner is a modification of GreatBay’s silk spinning machine. The parts we used were from the documentation they provided—extremely simple and easy to follow and freely available on their Hardware page
References
Amirnia, Shahram & Ray, Madhumita & Margaritis, Argyrios. (2015). Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor–biosorption system. Chemical Engineering Journal. 264. 863-872. 10.1016/j.cej.2014.12.016.
Carlsson, B. (2009). An introduction to modeling of bioreactors. http://www.it.uu.se/edu/course/homepage/modynsyst/vt09/Lecture/DynSystBior2009.pdf
Kai. Braukaiser, 25 Mar. 2013, http://braukaiser.com/blog/blog/2013/03/25/stir-speed-and-yeast-growth/.
Mazzoleni, S., Landi, C., Cartenì, F. et al. A novel process-based model of microbial growth: self-inhibition in Saccharomyces cerevisiae aerobic fed-batch cultures. Microb Cell Fact 14, 109 (2015). https://doi.org/10.1186/s12934-015-0295-4
Wong BG, Mancuso CP, Kiriakov S, Bashor CJ, Khalil AS. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER. Nat Biotechnol. 2018 Aug;36(7):614-623. doi: 10.1038/nbt.4151. Epub 2018 Jun 11. PMID: 29889214; PMCID: PMC6035058.









