Project

Human Practices

Team

Collaboration & Communication

Collaboration & Communication

Results
Overview
The experiments we have done during this year can be separated
into four main steps: The first step was the cloning and the confirmation of the E. coli strains for the G. algens
dextranase's expression
(BBa_K3493000). Secondly, we have optimized the proteic expression of our dextranase by
testing multiple conditions and thus purified the protein. Thirdly, we realized
micro-SNT enzymatic assays with the previously purified dextranase to characterize its
main properties as pH and temperature for optimal activity. Finally, rheological assays
allowed us to quantify the activity of our dextranase in ropy syrup, an essential test
for the proof of concept for this project. As a part of our contribution to a registry
part, experiments were also performed for the S. mutans
dextranase (BBa_K2551003).
1. Cloning and strains’ confirmation

Figure 1: Agarose 1% gel with the
diagnostic PCR products for three DH5alpha colonies from the transformation plate.
The transformed plasmid was pET28a-S.mutans. The PCR amplified the full length of
the coding sequence. The expected length was 2900pb. However, we observe that a
colonie expressed a truncated version of the plasmid.

Figure 1: Agarose 1% gel with the
diagnostic PCR products for three DH5alpha colonies from the transformation plate.
The transformed plasmid was pET28a-S.mutans. The PCR amplified the full length of
the coding sequence. The expected length was 2900pb. However, we observe that a
colony expressed a truncated version of the plasmid.
2. Proteins’ expression and purification
For both dextranase, G. algens and
S. mutans, expression’s
optimization experiments were performed to identify the optimal expression condition in
the BL21 strains. The parameters tested were the concentration of the inductor (IPTG),
the induction time and incubation temperature. For each condition and each dextranase,
an aliquot of the induced cultures was collected at different time points: at induction,
after 3h30 of induction and after 18h of induction. Those aliquots were used to estimate
the proteins’ expression by migration of the total extracts on SDS-PAGE gels .

 First, the optimal expression’s condition for the dextranase from
G. algens is at a concentration of 0.4 mM or 1mM of IPTG and at an incubation
temperature of 18 °C for 18 hours of induction. The increase of expression over
induction time is revealed by the increase of the protein band corresponding to the
expected weight of the G.algens’ dextranase of 63,5 kDa. We observe that the expression
of the protein is similar in every condition at 37°C, even if there is no inductor in
the medium. This can be explained by the basal rate of expression of the BL21
strain1.
Indeed, the bacteria can express the protein on the expression vector without the
inductor agent but at a low rate. Therefore, we can deduce that 37°C with IPTG is not
optimal conditions for the overexpression because the expression rate is similar to the
basal rate of the strain. The maximal expression observed in our experiment is at an
incubation temperature of 18°C. The lower temperature slowed the growth of E. coli, but
allowed a greater expression of the dextranase because it reduces synthesis rate thus
allowing the protein time to fold properly and avoiding aggregation2.
Moreover, another
way to explain the increase of expression at 18°C is the nature of G. algens which is a
psychrophilic bacteria. The optimal growth temperature for G.
algens is from 10°C to
12°C 3, therefore the induction temperature of 18°C is closer to the native
temperature
in which the dextranase is naturally expressed. In order to determine which condition
between 0.4mM and 1mM of IPTG is the best for induction, we performed a migration of the
soluble fractions and those results will be discussed later.
First, the optimal expression’s condition for the dextranase from
G. algens is at a concentration of 0.4 mM or 1mM of IPTG and at an incubation
temperature of 18 °C for 18 hours of induction. The increase of expression over
induction time is revealed by the increase of the protein band corresponding to the
expected weight of the G.algens’ dextranase of 63,5 kDa. We observe that the expression
of the protein is similar in every condition at 37°C, even if there is no inductor in
the medium. This can be explained by the basal rate of expression of the BL21
strain1.
Indeed, the bacteria can express the protein on the expression vector without the
inductor agent but at a low rate. Therefore, we can deduce that 37°C with IPTG is not
optimal conditions for the overexpression because the expression rate is similar to the
basal rate of the strain. The maximal expression observed in our experiment is at an
incubation temperature of 18°C. The lower temperature slowed the growth of E. coli, but
allowed a greater expression of the dextranase because it reduces synthesis rate thus
allowing the protein time to fold properly and avoiding aggregation2.
Moreover, another
way to explain the increase of expression at 18°C is the nature of G. algens which is a
psychrophilic bacteria. The optimal growth temperature for G.
algens is from 10°C to
12°C 3, therefore the induction temperature of 18°C is closer to the native
temperature
in which the dextranase is naturally expressed. In order to determine which condition
between 0.4mM and 1mM of IPTG is the best for induction, we performed a migration of the
soluble fractions and those results will be discussed later.

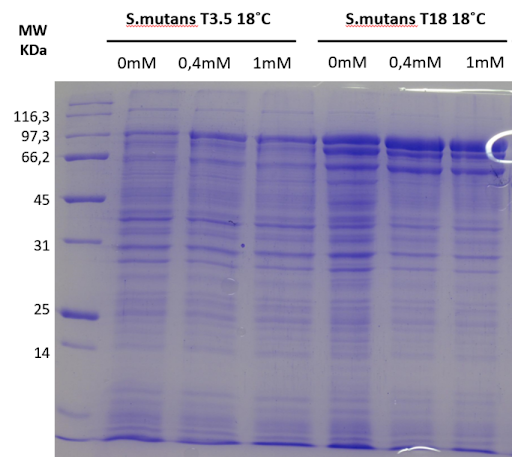 Furthermore, we were also able to express the dextranase of S.
mutans with the pET28 vector in the BL21 strain. It appears that the optimal expression
conditions for this protein could be at 1mM IPTG, 37°C for 3.5h or at 0.4/1mM IPTG at
18°C for 18h. However, the Comassi staining reaches saturation quickly, so we can not
tell with certainty which condition is the best for expression, because the 3 conditions
described above showed similar staining. For that reason, we have chosen to pursue the
expression’s condition of 0.4mM and 1mM IPTG at 18°C for 18h. As expected, we observe a
protein band at the expected molecular weight for this protein (95,4 kDa), but we also
see a lower weight band appear with the induction which could indicate protein
degradation. The degradation is more important at 18°C. To investigate this result, we
looked into the literature, because this protein had already been expressed and purified
prior to our experiments. In fact, this degradation was also observed by Kim et al.,
2011 4. They also report that the catalytic activity of both fragments is
still active.
Thus, we moved forward with the sonication and the purification for both dextranases.
Furthermore, we were also able to express the dextranase of S.
mutans with the pET28 vector in the BL21 strain. It appears that the optimal expression
conditions for this protein could be at 1mM IPTG, 37°C for 3.5h or at 0.4/1mM IPTG at
18°C for 18h. However, the Comassi staining reaches saturation quickly, so we can not
tell with certainty which condition is the best for expression, because the 3 conditions
described above showed similar staining. For that reason, we have chosen to pursue the
expression’s condition of 0.4mM and 1mM IPTG at 18°C for 18h. As expected, we observe a
protein band at the expected molecular weight for this protein (95,4 kDa), but we also
see a lower weight band appear with the induction which could indicate protein
degradation. The degradation is more important at 18°C. To investigate this result, we
looked into the literature, because this protein had already been expressed and purified
prior to our experiments. In fact, this degradation was also observed by Kim et al.,
2011 4. They also report that the catalytic activity of both fragments is
still active.
Thus, we moved forward with the sonication and the purification for both dextranases.

 As mentioned above, we used the cell pellets collected from the
optimization experiment (0.4mM & 1mM, 18°C T18) to perform sonication in order to assess
the solubility of the G. algens dextranase for both
conditions. In Figure 4A, we observe
that a larger amount of protein in the soluble fraction is visible for the 1mM condition
than for the 0.4mM condition. The difference between those conditions is the
concentration of IPTG, so we can believe that the higher concentration induced a larger
production of proteins that were not visible with the total extracts due to the Coomassie
staining saturation. With the soluble fraction of the protein expressed at 1mM IPTG 18°C
for 18 hours, we did a first test of purification with IMAC chromatography(Figure 4B).
The SDS-PAGE revealed a high-quality purification of our protein even if there is a loss
of dextranase during elution and washing. After all those verification steps, we
performed the expression experiment of G. algens dextranase
with the optimal condition
(Figure 3) at a larger scale in order to produce an important amount of dextranase. The
purity of this purification was similar to the one shown in Figure 4B, but for a higher
amount of proteins. Then, we quantified the concentration of purified protein with a
Bradford assay. We obtained a concentration of 4.01ug/uL of protein which equals to a
concentration of 636uM of the G.algens dextranase in our 15mL purified sample. We had
enough proteins to start the enzymatic assays. To our knowledge, this is the first time
the dextranase of G. algens is successfully expressed and
purified.
As mentioned above, we used the cell pellets collected from the
optimization experiment (0.4mM & 1mM, 18°C T18) to perform sonication in order to assess
the solubility of the G. algens dextranase for both
conditions. In Figure 4A, we observe
that a larger amount of protein in the soluble fraction is visible for the 1mM condition
than for the 0.4mM condition. The difference between those conditions is the
concentration of IPTG, so we can believe that the higher concentration induced a larger
production of proteins that were not visible with the total extracts due to the Coomassie
staining saturation. With the soluble fraction of the protein expressed at 1mM IPTG 18°C
for 18 hours, we did a first test of purification with IMAC chromatography(Figure 4B).
The SDS-PAGE revealed a high-quality purification of our protein even if there is a loss
of dextranase during elution and washing. After all those verification steps, we
performed the expression experiment of G. algens dextranase
with the optimal condition
(Figure 3) at a larger scale in order to produce an important amount of dextranase. The
purity of this purification was similar to the one shown in Figure 4B, but for a higher
amount of proteins. Then, we quantified the concentration of purified protein with a
Bradford assay. We obtained a concentration of 4.01ug/uL of protein which equals to a
concentration of 636uM of the G.algens dextranase in our 15mL purified sample. We had
enough proteins to start the enzymatic assays. To our knowledge, this is the first time
the dextranase of G. algens is successfully expressed and
purified.


Figure 2: SDS-PAGE 12% gels with full
extract aliquots from the expression optimization experiment of the G. algens
dextranase. The expected MW was 63.5kDa for the G.algens dextranase. We see the
increase in expression through induction time and the effect of temperature and IPTG
concentration on expression. The induction conditions in which the expression was
the best were 0.


Figure 3: SDS-PAGE 12% gels with full
extract aliquots from the expression optimization experiment of the S. mutans
dextranase. The expected MW was 95.4kDa for the full S.
mutans dextranase. However,
we observe multiple bands related to the induction which indicates proteolysis of
the protein of interest. We see the increase in expression through induction time
and the effect of temperature and IPTG concentration on expression. The induction
conditions in which the expression was the best were 0.4 mM or 1mM of IPTG and at an
incubation temperature of 18 °C for 18 hours of induction.


Figure 4: SDS-PAGE 12% gels showing
verification of solubility and purification of G. algens
dextranase. A. We compare
the soluble fraction induced with 0.4mM and 1mM IPTG to verify which allows a higher
concentration of the soluble enzyme. We observed that the 1mM condition confears a
larger amount of dextranase in the soluble fraction. B. Gel showing the steps of
IMACS purification. The purified protein seems to be quite pure. However, throughout
the purification we lose a lot of dextranase.
3. Enzymatic assays
Even though both enzymes, our designed dextranase from G. algens
(BBa_K3493000) and the one from S. mutans (BBa_K2551003),
were used for the previous
experiments, only the dextranase from G. algens was
characterised by the enzymatic
assays.
Prior to the micro-SNT enzymatic essays, the protocol was verified using a commercially available dextranase from Penicllium sp. (results not shown). We were able to confirm the linearity of the assay between 0.008 and 0.072 µmole of glucose equivalent as previously shown by Borkowska et al. 5. With the protocol adapted and functional, we measure the number of enzymatic units of the dextranase from G. algens. It revealed that for the equivalent of 0.002 µmole of dextranase, on the basis of amount of protein after purification, has 0.01 U at pH 6.5 and 18 °C. Here, the 1 U is equal to the release of 1 µmole of glucose equivalent after 1 min. This result show that the enzyme is active and retains its activity after lysis, purification and storage at 4 °C. Since this number of enzymatic units added to 0.4 % dextran leaded to the release of glucose equivalent in the linear region of the assay after 5 min of reaction at 18 °C, 0.01 U was used for the later enzymatic assays.

The pH optima were determined using the same method as the measurement of enzymatic units, but with variations of the pH of the dextran solution. From the activity’s results measured at pH ranging from 5.5 to 7.0 (Figure 5), the optimal pH of the G. algens’ dextranase is 6,5. Even though 6.5 is the optimal pH based on our results, the enzyme is still very active at 6.0 which is promising knowing that ropy maple is slightly acidic 6. On the other hand, the pH stability was similarly obtained with prior incubation of the dextranase at 100 µM in buffer ranging from pH 5.5 to 7 for 15 min at 18 °C. The better condition for the dextranase stability was attained at pH 7.0, but there is no intense fluctuation in the dextranase’s stability at slightly acidic pH. In comparison to the amount of released equivalent of glucose, either the enzymatic activity and stability measured between pH 5.5 and 6.5 are quite stable between 0.030 and 0.040 µmole. Knowing that the G. algens dextranase is slightly impacted in terms of stability at slightly acidic pH where the enzyme has a maximum activity, we can confirm that the enzyme is appropriate and functional on the basis of the pH for a treatment in the ropy maple syrup.

Using the same methodology as previously to measure the optimum activity with temperatures between 5 and 50 °C instead, we were able to determine that the temperature optimum for the activity of the enzyme is at 40 °C (Figure 6A). Since the treatment that we propose would be implemented at temperatures between 15 and 20 °C (see Implementation), we can see that even though the activity is about one-third of the activity at 40 °C, the enzyme is still very active. This suggests that, while the enzyme will not degrade the dextran at full capacity, this activity could be enough to significantly reduce the viscosity of ropy maple syrup to something comparable to maple syrup on an extended duration of treatment. After 15 min of incubation at each temperature followed by enzymatic assay at 18 °C for 5 min, we measured that the enzyme is at its highest stability at 15 °C and lower (considering the aberrant stability at 10 and 5 °C) (Figure 6B). This suggests that keeping the enzyme at 4 °C is enough to conserve its stability, which is promising for further uses in the industry without requiring specific and costly conservation conditions. This also shows that a treatment between 15 and 20 °C would be possible knowing that the enzyme would be close or at its peak stability but with an activity about one-third of its maximum, which is not problematic if the treatment is made for an extended period of time.
The enzymatic characterization we have made of the G. algens dextranase is the first time to our knowledge that the enzymatic properties of this enzyme were evaluated. Those results are essential for the development of the knowledge and the use of this dextranase from G. algens. Based on our results, we are confident that our dextranase is adapted to the situation of ropy maple syrup which requires either an enzyme active and stable at pH around 6.0 and 6.5 and an enzyme active and stable at temperature between 15 and 20 °C.
Prior to the micro-SNT enzymatic essays, the protocol was verified using a commercially available dextranase from Penicllium sp. (results not shown). We were able to confirm the linearity of the assay between 0.008 and 0.072 µmole of glucose equivalent as previously shown by Borkowska et al. 5. With the protocol adapted and functional, we measure the number of enzymatic units of the dextranase from G. algens. It revealed that for the equivalent of 0.002 µmole of dextranase, on the basis of amount of protein after purification, has 0.01 U at pH 6.5 and 18 °C. Here, the 1 U is equal to the release of 1 µmole of glucose equivalent after 1 min. This result show that the enzyme is active and retains its activity after lysis, purification and storage at 4 °C. Since this number of enzymatic units added to 0.4 % dextran leaded to the release of glucose equivalent in the linear region of the assay after 5 min of reaction at 18 °C, 0.01 U was used for the later enzymatic assays.

Figure 5: Activity and stability of the
G. algens’ dextranase in function of the pH at 18 °C
using micro-SNT enzymatic
assays. A. Activity of the dextranase based on the amount of released glucose
equivalent from the dextran T40 under the 0.01 U at pH ranging from 5.5 to 7.0. We
observe that the optimal for the enzyme is at pH 6.5 with 0.036 released glucose
equivalent, but followed closely at pH 6.0 and 7.0 with respective released glucose
equivalent of 0.031 and 0.032. B. Stability of the dextranase under the incubation
at pH between 5.5 and 7.0 for 15 min. The condition where the enzyme seems to be the
more stable is at pH 7.0.
The pH optima were determined using the same method as the measurement of enzymatic units, but with variations of the pH of the dextran solution. From the activity’s results measured at pH ranging from 5.5 to 7.0 (Figure 5), the optimal pH of the G. algens’ dextranase is 6,5. Even though 6.5 is the optimal pH based on our results, the enzyme is still very active at 6.0 which is promising knowing that ropy maple is slightly acidic 6. On the other hand, the pH stability was similarly obtained with prior incubation of the dextranase at 100 µM in buffer ranging from pH 5.5 to 7 for 15 min at 18 °C. The better condition for the dextranase stability was attained at pH 7.0, but there is no intense fluctuation in the dextranase’s stability at slightly acidic pH. In comparison to the amount of released equivalent of glucose, either the enzymatic activity and stability measured between pH 5.5 and 6.5 are quite stable between 0.030 and 0.040 µmole. Knowing that the G. algens dextranase is slightly impacted in terms of stability at slightly acidic pH where the enzyme has a maximum activity, we can confirm that the enzyme is appropriate and functional on the basis of the pH for a treatment in the ropy maple syrup.

Figure 6: Activity and stability of the
G. algens’ dextranase in function of the temperature at
pH 6.5 using micro-SNT
enzymatic assays. A. The activity of the dextranase is based on the amount of released glucose
equivalent from the dextran T40 under the 0.01 U at temperatures ranging from 5 to 50
°C. The enzyme showed to be more active at higher temperatures, reaching 0.104 µmole
of released glucose equivalent at 40 °C and slipping down over 40 °C. At 20 °C, the
activity is lowered to 0.037 µmole. B. Stability of the dextranase under the
incubation at a temperature between 5 and 55 °C for 15 min. We observe that the enzyme
is more stable at lower temperatures, 15 °C and below. The stability seems to
decrease when incubated below 15 °C, but those results appear to be aberrant,
considering that protein stability reaches a plateau at lower temperatures than the
optimal.
Using the same methodology as previously to measure the optimum activity with temperatures between 5 and 50 °C instead, we were able to determine that the temperature optimum for the activity of the enzyme is at 40 °C (Figure 6A). Since the treatment that we propose would be implemented at temperatures between 15 and 20 °C (see Implementation), we can see that even though the activity is about one-third of the activity at 40 °C, the enzyme is still very active. This suggests that, while the enzyme will not degrade the dextran at full capacity, this activity could be enough to significantly reduce the viscosity of ropy maple syrup to something comparable to maple syrup on an extended duration of treatment. After 15 min of incubation at each temperature followed by enzymatic assay at 18 °C for 5 min, we measured that the enzyme is at its highest stability at 15 °C and lower (considering the aberrant stability at 10 and 5 °C) (Figure 6B). This suggests that keeping the enzyme at 4 °C is enough to conserve its stability, which is promising for further uses in the industry without requiring specific and costly conservation conditions. This also shows that a treatment between 15 and 20 °C would be possible knowing that the enzyme would be close or at its peak stability but with an activity about one-third of its maximum, which is not problematic if the treatment is made for an extended period of time.
The enzymatic characterization we have made of the G. algens dextranase is the first time to our knowledge that the enzymatic properties of this enzyme were evaluated. Those results are essential for the development of the knowledge and the use of this dextranase from G. algens. Based on our results, we are confident that our dextranase is adapted to the situation of ropy maple syrup which requires either an enzyme active and stable at pH around 6.0 and 6.5 and an enzyme active and stable at temperature between 15 and 20 °C.
4. Rheological assays
After characterizing the enzymatic properties of G. algens’
dextranase, we were able to make the proof of concept of the project. Using ropy maple
syrup provided by the PPAQ, the organisation in charge of the market and quality
regulation of maple syrup. We measured the reduction of viscosity of ropy syrup under
the action of our dextranase using rheological assays.

To measure the reduction of viscosity, we incubated prior to the analyses ropy maple syrup with the dextranase at 35.5 µM for 50 minutes at ambient temperature. In comparison with the measured viscosity of ropy maple syrup with added buffer (Figure 7), we can see that the sample of ropy syrup under the action of the dextranase has a decreased viscosity when measured at 20 °C (Figure 6A). This suggests that not only the enzyme is active in ropy maple as estimated by the enzymatic assays, but the enzyme is able to significantly reduce its viscosity. To have a better view of the viscosity reduction after incubation since the relation between viscosity and temperature is exponential 7, we have done the same analysis at 10 °C with a prior incubation this time for 25 min at ambient temperature (Figure 6B). Again, we observed that treated ropy maple syrup shows a reduced viscosity compared to ropy maple syrup with buffer. This makes the proof of concept of our project, which is to reduce the viscosity of ropy syrup for it to be used as a sugar base.
Interestingly, when measuring the viscosity of ropy syrup alone (Figure 6A), we observed that the viscosity increases during the sampling time with more important fluctuations. We thought that the high concentration of sugar and dextran impacted the behaviour of the sample during the analysis. We think that crystallization of sugar might be responsible for this changing behaviour throughout the time of the rheological assay, but no further investigation was done since this behaviour was not observed when adding buffer as a control for the experiment.

Figure 7: Viscosity of ropy maple syrup
with and without added buffer and G. algens’ dextranase
using rheological assays.
The assays were performed at 20 °C and 10 °C with prior incubation of the ropy syrup
with the dextranase. The control of the viscosimetric measurement was done with the
same amount of buffer added to the ropy syrup. At 20 °C, the ropy syrup mixed buffer had a viscosity of 0.25 Pa•s while this value was 0.22 Pa•s when mixed with dextranase. The ropy
syrup alone increases in viscosity with increasing fluctuations during the sampling
time. At 10 °C, the ropy syrup mixed buffer had a viscosity of 0.47 Pa•s while this value was 0.42 Pa•s when mixed with dextranase.
To measure the reduction of viscosity, we incubated prior to the analyses ropy maple syrup with the dextranase at 35.5 µM for 50 minutes at ambient temperature. In comparison with the measured viscosity of ropy maple syrup with added buffer (Figure 7), we can see that the sample of ropy syrup under the action of the dextranase has a decreased viscosity when measured at 20 °C (Figure 6A). This suggests that not only the enzyme is active in ropy maple as estimated by the enzymatic assays, but the enzyme is able to significantly reduce its viscosity. To have a better view of the viscosity reduction after incubation since the relation between viscosity and temperature is exponential 7, we have done the same analysis at 10 °C with a prior incubation this time for 25 min at ambient temperature (Figure 6B). Again, we observed that treated ropy maple syrup shows a reduced viscosity compared to ropy maple syrup with buffer. This makes the proof of concept of our project, which is to reduce the viscosity of ropy syrup for it to be used as a sugar base.
Interestingly, when measuring the viscosity of ropy syrup alone (Figure 6A), we observed that the viscosity increases during the sampling time with more important fluctuations. We thought that the high concentration of sugar and dextran impacted the behaviour of the sample during the analysis. We think that crystallization of sugar might be responsible for this changing behaviour throughout the time of the rheological assay, but no further investigation was done since this behaviour was not observed when adding buffer as a control for the experiment.
Conclusion
In summary, we were able to express and purify our dextranase from G. algens using E.
coli BL21. We used the synthetic gene inserted in a conventional pET28a vector for
protein expression followed by IMAC chromatography. The purified enzyme was
characterized using micro-SNT enzymatic assays that revealed, in the condition of ropy
maple syrup (pH 6.5 and temperature between 15 and 20 °C), that the dextranase would be
adapted for the treatment. It was also confirmed using rheological assays.
References
- Dubendorff, J. W., & Studier, F. W. (1991). Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol, 219(1), 45-59. doi:10.1016/0022-2836(91)90856-2
- Rosano, G. L., & Ceccarelli, E. A. (2014). Recombinant protein expression in Escherichia coli: advances and challenges. Frontiers in Microbiology, 5(172). doi:10.3389/fmicb.2014.00172
- Bowman, J. P., McCammon, S. A., Brown, J. L., Nichols, P. D., & McMeekin, T. A. (1997). Psychroserpens burtonensis gen. nov., sp. nov., and Gelidibacter algens gen. nov., sp. nov., psychrophilic bacteria isolated from antarctic lacustrine and sea ice habitats. Int J Syst Bacteriol, 47(3), 670-677. doi:10.1099/00207713-47-3-670
- Borkowska, M., Białas, W., Kubiak, M., & Celińska, E. (2019). Rapid micro-assays for amylolytic activities determination: customization and validation of the tests. Applied microbiology and biotechnology, 103(5), 2367-2379. doi:10.1007/s00253-018-09610-0
- Lagace, L., Camara, M., Leclerc, S., Charron, C., & Sadiki, M. (2018). Chemical and microbial characterization of ropy maple sap and syrup.
- Andrade, E. N. D. C. (1930). The Viscosity of Liquids. Nature, 125(3148), 309-310. doi:10.1038/125309b0

