Description
The problem
2′-Fucosyllactose (2’-FL), one of the most abundant oligosaccharides in human milk, has potential applications in foods due to its health benefits such as the selective promotion of bifidobacterial growth and the inhibition of pathogenic microbial binding to the human gut. 2'-FL not only serves as a food ingredient added in infant formula, but also as a dietary supplement and medical food material in food bioprocesses. There is considerable commercial interest in 2'-FL for its irreplaceable nutritional applications[1]. Although 2`-FL has been produced industrially and successfully introduced into formula milk products, 2`-FL remains difficult to obtain at a reasonable price.The first reason is the scarce contents of 2`-FL in human milk, it is prohibitively expensive to obtain 2`-FL directly from human milk. Besides, the chemical method for 2`-FL production on the industrial scale is not very practical, because of its low stereoselectivity and the usage of toxic solvents. While the chemo-enzymatic approach might be mainly limited by the required enzymes and costly substrates.
Our Inspiration
With the rapid development of metabolic engineering and synthetic biology strategies, using the engineered cell factory for 2'-FL large-scale production might be a promising approach. From an economic and safety point of view, microbial selection for cell factory engineering in 2'-FL bioprocess also should be taken into consideration. When we talked with professor Chen from China Agricultural University, we know that to date, microbial production of 2’-FL has been studied mostly in Escherichia coli. And after we researched synthetic biologic literature, we found that through a fed-batch fermentation using engineered E.coli harboring the salvage pathway genes, 2’-FL was produced at a concentration of 23.1 g/L with yields of 0.367 mol 2’-FL/mol lactose and 0.355 mol 2’-FL/mole fucose[2]. But there are critical issues when producing 2’-FL by E.coli. First, there are endotoxin contamination in the produced 2’-FL. It is not good for food safety. Second, there are bacteriophage infection in the fermentation process to produce 2’-FL using engineered E.coli. After market research, we learned that both sellers and customers attach great importance to the problem of food safety. Therefore, we need to consider using a different type of microbe to produce 2’-FL, which is more safe.
Our Solution
Our solution is to produce 2’-FL by engineered Saccharomyces cerevisiae which is generally recognized as safe (GRAS) and has been widely used in food and pharmaceutical industries. Yeast have an advantage over bacteria in not being susceptible to phage infections that can be crippling in a manufacturing environment. And unlike E. coli, yeast doesn’t catabolize lactose, reducing the risk of yield loss.
Our Design
To produce 2’-FL in engineered S. cerevisiae using l-fucose and lactose as substrates, at least three genetic modifications must be achieved. First, l-fucokinase/GDP-l-fucose phosphorylase (FKP) needs to be introduced to produce intracellular GDP-l-fucose as a substrate of fucosyltransferase. It can use fucose as a substrate to produce GDP-l-fucose by two steps(figure1). We use promoter GAP, a strong yeast expression promoter derived from glyceraldehyde-3-phosphate dehydrogenase to promote the expression of FKP(figure2).

Figure 1. Using FKP to produce GDP-1-Fucose.

Figure 2. Using GAP promoter to promote the expression of FKP.
Second, lactose, a fucose acceptor in 2-FL production, needs to be transported into the cytosol of S. cerevisiae cells. Lactose permease(Lac12) from Kluyveromyces lactics needs to be introduced into S. cerevisiae for transporting lactose into the cytosol because wild-type S. cerevisiae is incapable of transporting lactose into the cytosol(figure3). We use TEF1 promoter, the yeast transcription elongation factor promoter to promote the expression of LAC12(figure4).
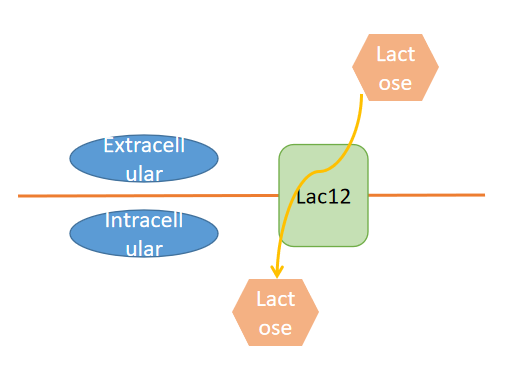
Figure 3. LAC12 transport lactose into the cytosol.

Figure 4. Using TEF1 promoter to promote the expression of LAC12.
Finally, α-1,2-fucosyltransferase, which catalyzes fucosylation of lactose into 2’-FL using GDP-l-fucose needs to be introduced into S. cerevisiae(figure5). Several α-1,2-fucosyltransferases have been verified to facilitate the synthesis of 2’-FL, which included FucT2 from Helicobacter pylori. We use ADH1 promoter, the yeast promoter for Alcohol Dehydrogenase I to promote the expression of FucT2(figure6).

Figure 5. FucT2 catalyzes fucosylation of lactose into 2’-FL using GDP-l-fucose.

Figure 6. Using ADH1 promoter to promote the expression of FucT2.
Above all, schematic representation of 2′-fucosyllactose production by engineered yeast Saccharomyces cerevisiae. Its the schematic diagram(figure7).

Figure 7. Producing 2’-FL by Saccharomyces cerevisiae.
In order to maintain the stability of the genes and prevent the loss of plasmids, while taking into account the yield and continuous production issues in the later fermentation process, we integrate genes “pGAP-FKP-pADH1-FucT2-pTEF1-LAC12” and the resistance gene AurR into the genome of Saccharomyces cerevisiae BY4741 for expression(figure8).

Figure 8. Integrate genes into the genome of Saccharomyces cerevisiae BY4741
Our Experiment
1.Construct recombinant plasmids. Get GAP promoter from vector PML104. Get TEF1 promoter from the genome of Saccharomyces cerevisiae BY4741. Get ADH1 promoter from vector pAUR123. Company synthetic genes of FKP, LAC12 and FucT2. Use vector pAUR123 to construct our plasimd “pAUR123-pGAP-FKP-pADH1-FucT2-pTEF1-LAC12”(figure9).

Figure 9. The plasimd “pAUR123-pGAP-FKP-pADH1-FucT2-pTEF1-LAC12”
2.Transform the product (2.5μL) into DH5α competent cells (50μL), grow cells on agar plates (containing Ampicillin). Incubate plates at 37°C overnight. Colonies were screened by colony PCR and then grown at 37℃, 200rpm. Plasmids were extracted and sent for sequencing.
3. PCR the genes “pGAP-FKP-pADH1-FucT2-pTEF1-LAC12” and the resistance gene AurR from the plasmid with homology arms of BY4741. Transform it into BY4741 by lithium acetate conversion method to integrate genes into the genome of BY4741 for expression. Screen for transformants by AbA-YPD selection medium.
4. Extract yeast total protein. Use SDS-PAGE to test whether the three proteins(FKP, LAC12, FucT2) are successfully expressed.
5. Fermentation to produce 2’-FL, 30 °C and 250 rpm. Use GC/MS and LC/MS to identify 2’-FL. Use HPLC to detection the yield of 2’-FL.
Our Goal
1.Successfully construct recombinant plasmids.
2.Successfully co-express three proteins(FKP, LAC12, FucT2).
3.Successfully produce 2’-FL.
The Further Application
2’-FL has been identified as a promising prebiotic in human milk, and economical and safe production of 2-FL on a large scale is highly desirable for various applications in infant formula and foods. S. cerevisiae has been widely used as a host for producing pharmaceuticals and nutraceuticals due to its GRAS status and well-developed genetic tools. After we successfully producing 2’-FL, we want to explore better fermentation conditions to increase the yield.
We expect our project to be applied to industrial production. According to our human practice survey, more and more parents, who are willing to feed their children with manual milk products rather than genuine human milk, are emerging in society( only 10% in 2016, increase to 64% in 2021). These parents are seeking for high-quality and low-price alternatives for human milk due to the inconvenience of breastfeeding their children and the ascending health assurance of these products. However, 2’-FL, which is the crucial and vital component of human milk, has not yet been artificial produced in China. Due to the lack of this ingredient, Chinese milk powder is not nutritious enough to keep newborn children healthy. We children born in big city such as Beijing can drink imported milk powder, which has 2’-FL to make our intestines healthier. But those children born in remote rural areas cannot drink nutritious milk powder, so they may have diarrhea and are not as healthy as we are. Therefore, we try our best to produce harmless 2’-FL in a cheaper and more efficient way. So we can produce high-nutrient-contained, high-quality, and low-price Chinese milk powder, which is benefit to the healthy growth of newborn children across China, not just us born in big city. In addition, there are many newborn children lack nutritious food in the world. If we can success, we hope children all over the world can grow up healthily because of this milk powder.
References
[1]Zhu Y, Wan L, Li W, et al. Recent advances on 2'-fucosyllactose: physiological properties, applications, and production approaches [published online ahead of print, 2020 Dec 1]. Crit Rev Food Sci Nutr. 2020;1-10.
[2]Chin YW, Kim JY, Kim JH, Jung SM, Seo JH. Improved production of 2'-fucosyllactose in engineered Escherichia coli by expressing putative α-1,2-fucosyltransferase, WcfB from Bacteroides fragilis. J Biotechnol.

