<!DOCTYPE html>

Hong Kong JSS

The Michaelis constant (Km) is defined to be the substrate concentration at which the reaction rate is half of maximum rate at standard temperature and pressure. Catalytic rate constant (kcat) is defined as the maximum number of chemical reactions converting substrate to product in standard time. Km and kcat of TVlac and FDR-A were identified by literature search. Km and kcat for FDR-A on aflatoxin were found to be 47μM and 63min-1 respectively (Taylor, 2010) . Though TVlac was well known to degrade aflatoxin, the kcat for such reaction cannot be found in literature. However, Km and kcat for TVlac on ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) were identified and used for graph generation. (Km: 38μM; kcat: 446.7min-1)


In another research, the aflatoxin catalytic characteristics of TVlac and FDR-A homolog (Actinomycetales sp.) were identified by experimental assay. The reported aflatoxin catalytic rates for TVlac and FDR-A were 0.37μg h-1 mg-1 and 11mg h-1 mg-1 respectively.

The graph shows that the time required for FDR-A undergo aflatoxin degradation is much less than TVlac.

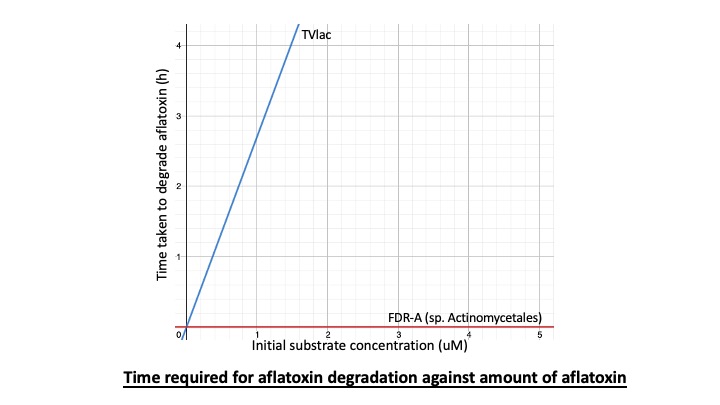
As a result, FDR-A is more preferred to be used in our final product. It is because it requires less time to degrade aflatoxin.
References:
Lyagin, I., & Efremenko, E. (2019). Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules, 24(13), 2362.
Taylor, M.C., Jackson, C.J., Tattersall, D.B., French, N., Peat, T.S., Newman, J., Briggs, L.J., Lapalikar, G.V., Campbell, P.M., Scott, C., Russell, R.J., Oakeshott, J.G., 2010. Identifica- tion and characterization of two families of F420H2-dependent reductases from Mycobacteria that catalyse aflatoxin degradation. Mol. Microbiol 78, 561–575.
Verheecke, C., Liboz, T., & Mathieu, F. (2016). Microbial degradation of aflatoxin B1: current status and future advances. International journal of food microbiology, 237, 1-9.
Wu, M. H., Lee, C. C., Hsiao, A. S., Yu, S. M., Wang, A. H. J., & Ho, T. H. D. (2018). Kinetic analysis and structural studies of a high‐efficiency laccase from Cerrena sp. RSD 1. FEBS Open bio, 8(8), 1230-1246.
Zeinvand-Lorestani, H., Sabzevari, O., Setayesh, N., Amini, M., Nili-Ahmadabadi, A., & Faramarzi, M. A. (2015). Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere, 135, 1-6.
