
Engineering success
Notice: This page is only a summary of our engineering success and the path to it. All the detailed information about the design, parts, experiments and results can be found at wiki pages Theory, Project Design, Wet lab and Results.
Research
For a large portion of our team, our iGEM journey started almost two years ago as we have decided to participate in iGEM 2020 as just the second team from Czech Republic ever. That year we decided to focus on cyanobacteria. As most of us had spent a lot of time in Brno - a city with a famous dam - water-bloom was a topic we were all familiar with and passionate about. That year we opted to design a system for directly reducing the population of cyanobacteria by lysing their cell wall and for the degradation of some of their toxins. While researching the topic of blue-green algae overabundance, one thing kept coming up - phosphate pollution of surface waters. As we discussed the topic further with various experts and even asked the general public (see more on page Human practices), it became clear that phosphate accumulation was truly a perfect direction for our project.
This year we want to target the cause of the problem instead of its symptoms. So instead of focusing on cyanobacteria themselves, we have opted to try and combat the increase in phosphate concentrations in water bodies. Phosphates are a vital source of nutrition for cyanobacteria and the large amounts of phosphate fertilizers which end up being washed from the fields into surface waters is one of the main contributors to the increasing incidence of water-bloom.
Another reason why phosphate accumulation is rather important is the ever increasing rate at which phosphates are used up - not just in agriculture but also in many different industries as well as consumer's homes. Phosphates are a finite resource which we will not be able to replace with an alternative any time soon. So the recycling of accumulated phosphates has definitely been one of our priorities.
Imagine
So now we had a goal in mind and it was finally time to think about the specifics.
When attempting to accumulate phosphates in bacteria - or any other organism for that matter - one must keep in mind that every living thing needs phosphates to live and thus has some way to intake and store this substance. That can be equally an advantage and a disadvantage. On one hand, we can be almost certain that our bacteria will take in phosphates from its surroundings even without our involvement. On the other hand, these phosphates will not stay inertly floating inside of its cytoplasm forever - they will be used to produce a variety of molecules and structures which in turn could be re-metabolised and phosphates could be released outside again.
BMCs
So in order to increase the rate at which our bacteria of choice retains phosphates, we have decided to use bacterial microcompartments or BMCs - large proteinous structures which encapsulate a biochemical pathway to provide ideal conditions for it, and to stop external influences. Phosphates should be able to easily pass through the semipermeable wall of the BMC where it would be incorporated into a larger structure which would no longer be able to get outside.
To store phosphates in larger molecules, we have decided to encapsulate the enzyme polyphosphate kinase or PPK into the BMCs. This enzyme links phosphates into large chains called polyphosphates. Polyphosphates in BMCs would be safe from being digested by other enzymes and would thus remain in our system and would not reenter into circulation.
PPK can be easily localized to BMCs by fusing this protein to a couple of N-terminal amino acids from one of the proteins which are naturally encapsulated in these microcompartments.
Response to phosphate concentrations
BMCs sounded like an ideal means of improving phosphate accumulation. But there was another issue. BMC production requires the expression and assembly of large amounts of heterologous proteins and can thus decrease the vitality of modified bacteria. This issue could be partially solved by ensuring that BMC expression would only be triggered under specific conditions. Making BMC production tied into the changes in phosphate levels was an obvious solution.
As mentioned above, all organisms need to take in phosphates. That means that all organisms must have a way of responding to changes in phosphate concentrations. In many other bacteria, this task is performed by the Pho pathway, where the phosphorylation (or lack thereof) of the PhoR receptor leads to changes in gene expression from the PPho promoter. This pathway is activated by a lack of phosphates and downstream of this promoter are thus located genes which mediate phosphate intake and ensure that the bacteria gets enough of this vital nutrient. So by placing a repressor of a promoter controlling BMC production under the PPho promoter native to the bacteria, we can create a system which responds to higher phosphate concentrations by producing BMCs with encapsulated polyphosphate kinase.
Bacillus subtilis
One of the easier decisions was choosing an organism to work with. Last year we opted for Bacillus subtilis and this year we decided to not break that pattern. As opposed to E. coli, B. subtilis can thrive in lower E. coli temperatures of water bodies while also producing a decent amount of proteins. It has also been already demonstrated that heterologous BMC production in these bacteria is possible and the Pho pathway and the sequences of its genes were also documented thoroughly for this organism.
More details about the theory behind our project and our ideas can be found on the page THEORY.
Design
So now we had an idea of how our system should look and what genes we needed to express in our modified B. subtilis. It was the time to design and pick our parts and carefully put them together into composite parts which would not only serve our purpose but also allow us to test their functionality and visualize what is happening in our system. We have designed four composite parts to test various components of our system. Parts of three of these constructs can be assembled together later on to form a functioning system which responds to the increase in phosphate concentrations by BMC production. The last construct was designed for the production of a protein which would be encapsulated in these BMCs.
More detail about each composite part can be found on the page PROJECT DESIGN.
BMC production and protein encapsulation
Composite part A was designed to test and characterise the production and assembly of BMCs in Bacillus subtilis. We opted for the heterologous production of BMC shell proteins from the Pdu operon of the bacteria Parageobacillus thermoglucosidasius which is closely related to B. subtilis. Heterologous production and assembly of Pdu BMC in B. subtilis has been demonstrated in a study by Wade et al. (2019), where they have identified five essential shell proteins. In composite part A, we place all five essential Pdu BMC shell proteins downstream of an IPTG inducible promoter in order to effectively induce BMC production without fearing that it would drastically decrease the vitality of our bacteria. This promoter would later on be replaced by a modified promotor from composite part D, which should be able to bind cI repressor and thus stop the expression of BMCs in the presence of this molecule.

Figure 1:
Composite part A
In that study, they have used 24 N-terminal amino acid residues from PduP protein as a tag to encapsulate protein inside of Pdu BMCs. PduP tag binds to C-terminal helixes of shell proteins of BMC and mediates incorporation of fused protein, in our case GFP, into the BMC. In composite part B, we have fused this PduP tag with the green fluorescent protein GFP. The localization of the fluorescent signal into the BMC, instead of it being dispersed through the entire cell, should thus indicate correct assembly of BMCs as well as the encapsulation of protein on the inside.

Figure 2:
Composite part B
The fluorescent protein GFP could be replaced by PPK enzyme further down the line to create a functional system for sequestering and storing phosphates in BMCs in a form of polyphosphates.
Response to phosphate concentration
Composite part C was designed to test the response of PPho promoter in Bacillus subtilis to the changes in phosphate levels in the medium. As mentioned above PPho is a native promoter of B. subtilis and it is activated by the Pho signaling pathway which is in turn active in low phosphate concentrations. This promoter was described in a study by Hulett et. al. We used the predicted sequence from this study and placed a reporter protein mScarlet-I downstream of this promoter to monitor its function. The cI repressor has also been placed under the control of PPho promoter.

Figure
3: Composite part C
In low phosphate concentrations in minimal medium, modified bacteria should produce mScarlet-I and fluorescent signal should be present. In high phosphate concentrations, the promoter should be inactive and no fluorescent signal should be measured.
Modified promotor and its repression
There are not many efficiently repressible promoters which would also work well in B. subtilis. To create our switch system, we opted to modify the Pgrac promoter. Original Pgrac can be repressed by lacI repressor which can be removed from the operator of this promoter by IPTG. We took out the lacO sequence and replaced it by the cI operator (OcI) sequence. This design should allow the strong promoter to constitutively initiate transcription until the cI repressor binds to OcI and the expression is stopped.
Repressor cI is natively produced by phage lambda where it controls the decision between initiating lytic or lysogenic cycle. This repressor binds to the O1, O2 and O3 operators, forms multimers and bends the DNA molecule, preventing transcription initiation from the pR promoter. We added one of the operator sequences to the Pgrac promoter to create Pgrac-OcI which should thus be repressed by the cI repressor. As cI repressor is a peptide, it can be easily produced by B. subtilis, allowing our system to regulate on its own.
To test the functionality of this switch system, we have placed sequences coding mScarlet-I and cI repressor downstream of the IPTG inducible Phyperspank promoter and GFP gene downstream of our modified Pgrac-OcI. All proteins were fused with SNV degradation tags to ensure that our system will be able to react quickly to induction and there would be no proteins which would remain in the cytoplasm long after their promoter was turned off.

Figure 4:
Composite part D
Without IPTG induction, cI repressor as well as the reporter fluorescent protein mScarlet-I is not produced so there is nothing stopping Pgrac-OcI from initiating transcription of GFP and we should thus be able to measure typical GFP fluorescence. After IPTG induction, the Phyperspank can initiate the expression of mScarlet-I and cI repressor. The repressor should bind to Pgrac-OcI and repress the production of GFP. After a while, there should be no or very little GFP fluorescence and a lot of signal coming from mScarlet-I.
Putting it all together
Composite part Z has been combined from previously mentioned parts A, C, and D to create a functional system which responds to increase in phosphate levels by initiation of BMC production and assembly.
This construct will be assembled by modifying composite part D using restriction digestion. Promoter Phyperspank would be replaced by the PPho promoter from composite part C which responds to the phosphate concentration in the growth medium. Additionally, the GFP sequence would be replaced by the sequences of the Pdu BMC shell proteins from composite part A. This modification would be done using restriction sites NdeI and XhoI.

Figure 5:
Composite part Z
At low phosphate concentrations, PPho promoter should activate the transcription of the cI sequence and lead to the production of the cI repressor. This repressor inhibits transcription initiation from the Pgrac Oc promoter and thus prevents the formation of more BMCs. This system ensures that BMCs are produced only when the bacteria encounter high phosphate concentrations.
Build
In our project we used plasmids pDG1664 and pDG3661. They are shuttle vectors, which means that they are designed to be used in two different organisms - in our case E. coli and B. subtilis. As they are designed for E. coli ectopic integration into the chromosome of B. subtilis, they only carry replication origin for E. coli. This ensures that we are able to store and replicate our plasmids in E. coli, while ensuring that colonies of B. subtilis which exhibit resistance to corresponding antibiotics should have the target sequence integrated into its chromosome.
Vector pDG1664 was utilized for composite part B. It carries a gene encoding resistance to ampicillin for selection in E. coli and a gene for resistance to erythromycin for selection of transformants in B. subtilis which is thus located in the segment which will be integrated into the chromosome of B. subtilis, more specifically into the thrC sequence.

Figure 6: Schematic depiction of pDG1664 plasmid
Vector pDG3661 was utilized for composite parts A, C, D and Z. It carries a gene encoding resistance to ampicillin for selection in E. coli and a gene for resistance to chloramphenicol for selection of transformants in B. subtilis which is thus located in the segment which will be integrated into the chromosome of B. subtilis, more specifically into the amyE sequence.
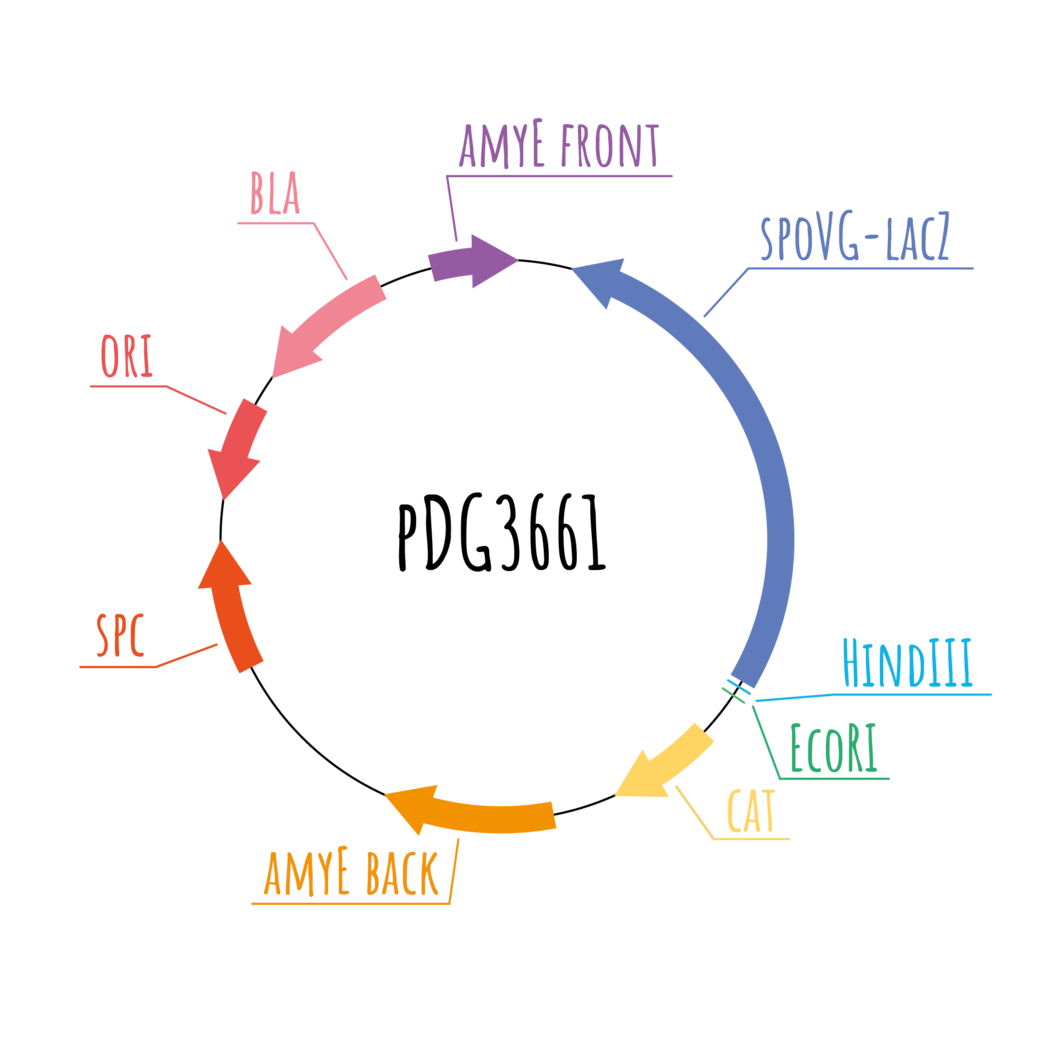
Figure 7: Schematic depiction of pDG3661
We ordered our constructs to be synthesized and delivered in plasmids from company IDT. They were sent to us in pUCIDT plasmids which contain a replication origin for E. coli and encode resistance to ampicillin.
When ordering the constructs for synthesis, we have encountered a big problem - composite part A had a high level of homology due to the similarities in sequences of Pdu shell proteins. We have opted to order the rest and then split composite part A into two constructs and change the design so it could be ligated into one construct with Golden Gate cloning. That has however taken a log of time as we needed to research the method first. In the end, the two halves of composite part A arrived too late and we did not have the time to work with it and achieve results.
Therefore, everything described below was only done with composite parts B, C and D.
Cloning into shuttle vectors and transformation of E. coli
First we transformed non-expression E. coli JM109 strain with pUCIDT plasmids containing our constructs to store and replicate these vectors.
Then we needed to clone our constructs into the vector mentioned above. We performed restriction digestion of our constructs in pUCIDT plasmids as well as the empty vectors using enzymes BamHI and HindIII. Correct size of fragments was verified using electrophoresis and the fragments were then cut out and purified from the gel.
Digested constructs were ligated with corresponding plasmids and were subsequently used for the transformation of chemocompetent E. coli cells which were then inoculated onto agar plates containing ampicillin. We have transformed two E.coli strains - E. coli JM109 and expression strand BL21(DE3), which has a gene for lacI inserted into its chromosome and can thus be used for IPTG induction. Transformation of E. coli was confirmed using restriction digestion and colony PCR.
Transformed JM109 E. coli cells were used for storage and replication of our plasmids. We made glycerol stocks of E. coli transformants to be kept at -80°C. We have inoculated some of the verified cultures into liquid LB media and let them grow overnight. Afterwards we performed plasmid isolation to obtain enough shuttle vectors pDG3661 and pDG1664 containing our constructs for transformation of B. subtilis and integration into its chromosome. Transformed BL21 (DE3) E. coli colonies were used to test the functionality of our composite parts.
Further information and results can be found on the page RESULTS in the section PREPARATION OF E. COLI CLONING HOST.
Transformation of Bacillus subtilis
We have transformed B. subtilis 168 cells with pDG1664 and pDG3661 plasmids containing our constructs which were isolated from E. coli JM109 colonies. Vector pDG1664 was designed for integration into the sequence of thrC and vector pDG3661 was designed for integration into the sequence amyE. A combination of antibiotics erythromycin and lincomycin were used for selection of successfully transformed colonies transformed with pDG1664_B vectors. The antibiotic chloramphenicol was used for selection of transformants containing pDG3661_D.
Successful transformation and the insertion into the chromosome of B. subtilis was verified by AmyE test and also by long PCR with one primer complementary to a sequence on the chromosome of B. subtilis in the proximity of the insertion site while the other primer annealed to our composite part. The amplicons containing a sequence spanning from the chromosome of B. subtilis to the beginning of our composite part were then sent for sequencing for further proof.
Further information and results can be found on the page RESULTS in the section INTEGRATION OF SYNTHETIC CONSTRUCTS INTO THE CHROMOSOME OF B. SUBTILIS.
Composite part C
There were some issues with cloning Composite part C into pDG3661 After many tries where the construct remained inside of the pUCIDT plasmid, we have decided to first amplify the sequence of construct C using long PCR and then perform restriction digestion and ligation using only the amplicon, hoping that it would not re-ligate to pUCIDT this time. This approach ended up working but transformation of construct C into E. coli and then B. subtilis ended up taking significantly more time and we did not have time to functionally characterize this construct.
If we had time to characterize composite part C we would have used the following approach. B. subtilis cells with construct C in the chromosome would be first grown in LB medium. The cultures would then be washed and inoculated into minimal MOPS media adjusted for B. subtilis and modified in a way where all phosphate components are replaced by Tris-HCl. Phosphates can be then added to the medium in varying amounts to create media with different phosphate concentrations. These cultures would then be pipetted onto plates and both OD and mScarlet-I fluorescence would be continuously measured using Tecan fluorometer. In low phosphate concentrations, the PPho should be turned on, mScarlet should be produced and the fluorescence intensity should increase.
Figure 8:
Scheme of our experimental approach
Test
So now that we had strains of both B. subtilis and BL21(DE3) E. coli containing each of our constructs, it was time to test out the functionality of our constructs. One issue we encountered was that the strain of B. subtilis which we were using did not contain the gene for lacI repressor and thus couldn't be used for IPTG induction without inserting this gene into its chromosome. At the beginning of our project, we assumed that lacI was already inserted into the chromosome of our B. subtilis lacI strain and we only found out otherwise later on. All of our IPTG inducible promoters thus worked as constitutive in our B. subtilis cells. It was too late to perform another transformation, so we decided to use the aforementioned expression strand of E. coli BL21(DE3) to test the response of our composite parts to IPTG induction.
Characterization of constructs B and D
After successful transformation of E. coli and B. subtilis, we designed the E. coli following experiments to demonstrate the functionality of the different components of our project design.
First we determined the growth curve for each culture from regular measurements of OD600. We tested how the system responds to the presence of IPTG in the expression strain of E.coli BL21(DE3) cells. In B. subtilis cells we presumed that IPTG inducible promoters would behave as constitutive.
We used several different methods to detect the presence of the reporter proteins GFP and mScarlet-I. We used a Tecan plate fluorometer for simultaneous and continuous measurement of both OD600 and fluorescence of the cultures. Additionally, we used FluoroMax Plus from Horiba Scientific for more sensitive measurement of fluorescence spectrum in cuvettes at one point in time. We then verified the results by fluorescence microscopy.
We should keep in mind that in B. subtilis, our constructs were integrated into its chromosome and thus only one copy was present in each cell. E.coli BL21(DE3) cells on the other hand contained our constructs inserted into multicopy pUCIDT plasmids. The expression of the target proteins was therefore much stronger in E.coli.
Table 1: E. coli cultures used in following experiments
|
Experiment |
Constructs |
Fluorescent proteins |
Induction (IPTG) |
Label |
|
E. coli BL21 (DE3) |
None - controls |
- |
- |
BL21(DE3) without IPTG |
|
At OD 0.6 |
BL21(DE3) IPTG 0.6 |
|||
|
At inoculation |
BL21(DE3) IPTG overnight |
|||
|
Composite part B |
GFP |
- |
BL21(DE3)_PUCIDT_B without IPTG |
|
|
At OD 0.6 |
BL21(DE3)_PUCIDT_B IPTG 0.6 |
|||
|
At inoculation |
BL21(DE3)_PUCIDT_B IPTG overnight |
|||
|
Composite part D |
GFP mScarlet-I |
- |
BL21(DE3)_PUCIDT_D without IPTG |
|
|
At OD 0.6 |
BL21(DE3)_PUCIDT_D IPTG 0.6 |
|||
|
At inoculation |
BL21(DE3)_PUCIDT_D IPTG overnight |
|||
|
E. coli BL21 (DE3) |
None - controls |
- |
- |
BL21(DE3) without IPTG |
|
At OD 0.8 |
BL21(DE3) IPTG |
|||
|
Composite part B |
GFP |
- |
BL21(DE3)_PUCIDT_B without IPTG 0.8 |
|
|
At OD 0.8 |
BL21(DE3)_PUCIDT_B IPTG 0.6 |
|||
|
Composite part D |
GFP |
- |
BL21(DE3)_PUCIDT_D without IPTG 0.8 GFP |
|
|
At OD 0.8 |
BL21(DE3)_PUCIDT_D IPTG 0.8 GFP |
|||
|
mScarlet-I |
- |
BL21(DE3)_PUCIDT_D without IPTG 0.8 mScarlet_I |
||
|
At OD 0.8 |
BL21(DE3)_PUCIDT_D IPTG 0.8 mScarlet_I |
Determination of growth curves by continuous measurements of OD by Tecan fluorometer
Firstly, we have visualized the growth of each of our cultures to determine if the expression of our target proteins of each of our constructs poses a metabolic burden on transformed cells.

Figure 9: Growth
curves of E. coli BL21 (DE3) strains
We can extrapolate from the growth curves that the E. coli BL21 (DE3) cultures grow more slowly after IPTG induction. This is particularly apparent in the cultures which were induced during inoculation. This demonstrates a slight toxicity of IPTG for our E. coli cultures as this effect was observed even in our controls to which IPTG was added.
The growth of cultures transformed with construct B was not significantly different from the growth of our controls, so it does not seem to carry increased metabolic burden. The growth of cultures transformed with construct D was slowed down as these cultures remained in the lag phase far longer than the controls.
Measurement of emission spectra of mScarlet-I and GFP in E. coli BL21(DE3) using FluoroMax Plus
We have also used fluorospectrometer FluoroMax Plus from Horiba Scientific, which is more sensitive and allows the measurement of signals with different wavelengths through the spectrum of light. The measurements can however not be done continuously and were thus performed at one point in time - 4 hours after IPTG induction.

Figure 10:
Emission spectra of GFP from construct B in E. coli BL21 (DE3)

Figure 11:
Emission spectra of GFP from construct D in E. coli BL21 (DE3)

Figure 12:
Emission spectra of mScarlet-I from construct D in E. coli BL21
(DE3)
Typical fluorescent signal profile of corresponding fluorescent protein as well as an increase in fluorescence intensity compared to the controls was recorded both in cultures carrying construct B (GFP signal) and construct D (sf GFP and mScarlet-I signals). This indicates that each fluorescent protein in corresponding constructs is being expressed and is functional.
There was however no significant difference in fluorescence intensity of cultures which were induced with IPTG and cultures where IPTG was not added. This is in accordance with the measurement of mScarlet-I relative fluorescence in the Tecan fluorometer. This further demonstrates that the IPTG inducible promoters are functioning constitutively.
Plasmids from the pETM line, which are most commonly used for the transformation of E. coli BL21 (DE3) typically contain an additional lacI gene to ensure that all inducible promoters are sufficiently repressed. We however didn't have these plasmids available on short notice and thus we used pUCIDT plasmids which do not carry the gene for lacI repressor. It is therefore possible that the cells were not able to produce sufficient amount of lacI repressor from one gene integrated into their chromosome to effectively stop the expression from our IPTG inducible promoter which were located on multicopy plasmids.
Moreover, if the expression from our IPTG inducible promoters is truly constitutive it would mean that in construct D, protein mScarlet-I as well as the repressor cI should be expressed continuously. Repressor cI should then repress the modified promoter PGrac-OcI which controls the expression of GFP. The fluorescence intensity of GFP from construct D is significantly lower than of GFP from construct B. This might suggest that our modified promoter can truly be somewhat repressed by the repressor cI. There are however other explanations why the expression from construct D would be lower - construct D is bigger and poses a larger metabolic burden on the cells so the cultures might not have had the same OD at the time of measurement. So this is only a small indication which requires further testing.
Observation of E. coli BL21(DE3) cultures under fluorescence microscope
Findings from previous measurements were confirmed using fluorescence microscopy. More information about our experimental setup can be found in the section WET LAB and RESULTS.

Figure 13: Induced E. coli BL21(DE3) cells transformed with construct B
under fluorescence microscope. Images of native unfixed samples of E. coli cells carrying construct B
induced with IPTG taken with a fluorescence
microscope. The image on the left is a brightfield image, the image in the
middle was taken with a GFP filter and on the right is the overlay of the
two. Immersion oil was used. M = 52x

Figure 14: Uninduced E. coli BL21(DE3) cells transformed with construct B
under fluorescence microscope. Images of native unfixed samples of E. coli
cells carrying construct B without IPTG induction taken with a fluorescence
microscope. The image on the left is a brightfield image, the image in the
middle was taken with a GFP filter and on the right is the overlay of the
two. Immersion oil was used. M = 52x

Figure 15:
Induced E. coli BL21(DE3) cells transformed with construct D under
fluorescence microscope. Images of native unfixed samples of E. coli cells
carrying construct D induced with IPTG taken with a fluorescence
microscope. The image on the left is a brightfield image, the following
image was taken with a GFP filter, the next image on the right was taken
with mScarlet-I filter and on the right is the overlay of the three.
Immersion oil was used. M = 52x
Table 2: B. subtilis cultures used in our experiments
|
Experiment |
Construct |
Fluorescent protein |
Induction |
Label |
|
Tecan fluorometer |
None - control |
- |
- |
B. subtilis 168 |
|
GFP gene in chromosome + control |
GFP |
Xylose induction |
B. subtilis positive control GFP in chromosome |
|
|
Composite part B |
GFP |
- |
B. subtilis _B |
|
|
Composite part D |
GFP mScarlet-I |
- |
B. subtilis _D |
|
|
FluoroMax Plus |
None - control |
- |
- |
B. subtilis 168 |
|
GFP gene in chromosome + control |
GFP |
Xylose induction |
B. subtilis positive control GFP in chromosome |
|
|
Composite part B |
GFP |
- |
B. subtilis _B |
|
|
Composite part D |
GFP |
- |
B. subtilis _D GFP |
|
|
mScarlet-I |
- |
B. subtilis _D mScarlet_I |
Determination of growth curves by continuous measurements of OD by Tecan fluorometer
Firstly, we have visualized the growth of each of our cultures to determine if the expression of our target proteins of each of our constructs poses a metabolic burden on transformed cells.

Figure 16: Growth
curves of B. subtilis 168 strains
The growth rate of cultures transformed with constructs B and D decreases down a little during the later phase of their growth compared to the controls. This indicates that both constructs pose a metabolic burden on B. subtilis 168 cultures.
Expression of fluorescent proteins from B. subtilis was a lot smaller than from E. coli and we were therefore unable to measure any fluorescent signal using the less sensitive Tecan fluorometer. Fluorescence in B. subtilis was later recorded using FluoroMax Plus.
Measurement of emission spectra of mScarlet-I and GFP in B. subtilis 168 using FluoroMax Plus
We have also used fluorospectrometer FluoroMax Plus from Horiba Scientific, which is more sensitive and allows the measurement of signals with different wavelengths through the spectrum of light. The measurements can however not be done continuously and were thus performed at one point in time - 4 hours after the xylose induction of positive control.

Figure 17:
Emission spectra of GFP from construct B in B. subtilis
In B. subtilis, typical fluorescent signal profile of corresponding fluorescent protein as well as an increase in fluorescence intensity compared to the negative controls was only recorded in cultures with construct B in their chromosome (GFP signal). This indicates that fluorescent protein GFP in construct B is being expressed and is functional.
There was however no significant increase in fluorescence intensity compared to the negative controls in both GFP and mScarlet-I in cultures with construct D in their chromosome. This is however contradicted by the observations made using fluorescence microscopy, so we cannot reach a concrete conclusion in regards to the expression of GFP and mScarlet-I from construct D in B. subtilis .
Observation of B. subtilis cultures under fluorescence microscope
B.subtilis strains with constructs B and D in their chromosome as well as
the control strain with no integration, were also observed using
fluorescence microscopy. More information about our experimental setup can
be found in the section WET LAB
and RESULTS.
Figure 18:
B. subtilis 168 cells with no integration in their chromosome under
fluorescence microscope. Images of native unfixed samples of B. subtilis
taken with a fluorescence microscope. This image shows a culture of B.
subtilis 168 with no insert in it's chromosome. The image on the left is a
brightfield image, the image in the middle was taken with a GFP filter and
on the right is the overlay of the two. Immersion oil was used. M = 52x
Figure 19: B. subtilis 168 cells transformed with construct B under
fluorescence microscope. Images of native unfixed samples of B. subtilis
cells taken with a fluorescence microscope. This image shows a culture of
B. subtilis _B. The image on the left is a brightfield image, the image in
the middle was taken with a GFP filter and on the right is the overlay of
the two. Immersion oil was used. M = 52x
Figure 20: B. subtilis 168 cells transformed with construct D under
fluorescence microscope. Images of native unfixed samples of B. subtilis
taken with a fluorescence microscope. This image shows a culture of B.
subtilis_D. The image on the left is a brightfield image, the following
image was taken with a GFP filter, the next image to the right was taken
with a mScarlet-I filter and on the right is the overlay of the three.
Immersion oil was used. M = 52x
When compared to the control - B. subtilis 168 with no insert in its chromosome - there is an indication of fluorescence in cultures transformed with both construct B and D. In culture with construct D in its chromosome there is an indication of both GFP and mScarlet-I fluorescence. This contradicts the measurements of emission spectra made with FluoroMax Plus and these results are thus inconclusive and need more experimal backing.
Summary
We have obtained the growth curves of both E. coli and B. subtilis cultures E. coli transformed with constructs B and D using Tecan fluorometer. While there were some apparent effects of higher metabolic burden on the growth of E. coli carrying construct B and B. subtilis with either construct in its chromosome, the presence of our constructs does not seem to be too detrimental to the culture.
We have managed to confirm the expression and functionality of all fluorescent proteins from constructs B and D in E. coli with measurements from Tecan fluorometer (only mScarlet-I), FluoroMax Plus and fluorescence microscopy.
In E. coli BL21(DE3), there was no difference in the expression of all fluorescent proteins from constructs B and D after IPTG induction. Both of our IPTG inducible promoters seem to be initiating transcription constitutively. This might be the result of lower expression of lacI repressor which is inserted in bacteria's chromosome compared to the number of IPTG inducible promoters which are present in the cell in multicopy plasmids.
Fluorescence intensity of GFP produced by E. coli carrying construct D was significantly lower than of GFP produced by E. coli carrying construct B. In construct D, GFP expression is controlled by PGrac-OcI promoter which was modified to respond to repressor cI. This repressor is also produced by construct D where it is placed downstream from an IPTG inducible promoter PHyperspank and its expression should thus roughly correspond to the expression of mScarlet-I which is also controlled by this promoter. So if IPTG inducible promoters are not properly repressed by lacI, the lower fluorescence intensity of GFP from construct D could be caused by the constitutive expression of repressor cI. This would indicate that our modified promoter can indeed be somewhat repressed by cI. This is however not conclusive and still requires further testing.
The expression and functionality of all GFP from constructs B in B. subtilis was also confirmed with measurements from FluoroMax Plus and fluorescence microscopy.
Fluorescence of GFP and mScarlet-I in B. subtilis cultures transformed with construct D was however only detected by fluorescence microscopy. It is thus still unclear whether the expression and correct folding of mScarlet-I and GFP is taking place in B. subtilis cultures transformed by construct D and further testing is necessary to confirm our findings.
Improve
Our project is far from being completed as there are many aspects which could be improved in the future.
One large part is the expression and assembly of BMCs which was not tested as we were not able to perform Golden Gate cloning of composite part A. One takeaway from this situation is to consider the level of homology between the sequences of parts which the constructs are composed of. If they are quite similar, it might be wise to split the composite part into multiple constructs designed to be joined by Golden Gate cloning or other such method straight away, so we would wait for the verdict of the company from which we order synthetic constructs.
As is, it is likely that IPTG induction would not work for any of our constructs. The functionality of IPTG induction in B. subtilis should be enabled by integrating the gene for lacI repressor controlled by a strong constitutive promoter into the chromosome of B. subtilis strains which already have our composite parts inserted into the chromosome. Transformation of B. subtilis with lacI gene could also be done using plasmids designed for stable expression in this bacteria. However, we do not have any experience working with them and chromosomal integration was recommended to us by experts working with B. subtilis. As both options have their pros and cons, it will most likely take more research to decide which would work the best for us.
In E. coli BL21(DE3) we can clone the constructs into a plasmid which is designed for IPTG induced expression in E. coli BL21(DE3) - such as the pET plasmids. These plasmids should contain an extra lacI gene to ensure the repression of our genes in the absence of IPTG and this could improve the functionality of IPTG induction in E. coli BL21(DE3).
When subcloning construct C from pUCIDT plasmid into pDG3661, we struggled to get rid of the contamination by pUCITD plasmid. This problem was largely caused by the fact that we have used the same selection marker for both plasmids, so colonies containing pUCIDT instead of pDG3661 could grow on our plate. This is definitely a mistake we will not repeat in the future. The functionality of construct C also remains to be tested. This construct does not require IPTG induction to function fully so we should be able to test it out without further transformation of B. subtilis strain with this construct in the chromosome.
Another aspect of our project to consider is that the level of expression of our reporter proteins from the chromosome of B. subtilis might not be as high as from multicopy plasmids. This might be a problem, especially for the expression and correct assembly of BMCs, as the heterologous expression of BMC was most commonly performed and studied in E. coli with plasmids carrying the genes of the shell proteins. In our experiments, the expression of GFP from B. subtilis strain transformed with construct B was however rather good so that might not be an issue. Additionally, overexpression can also harm the cell and slow down the growth of the culture, so having only one copy of each gene might actually be advantageous when producing culture which should remain alive and replicating inside of our device for some time.
We are well on the way to verifying the functionality of our project design. The time is however playing against us and we were thus unable to perform the optimization and improvements needed. We are looking forward to maybe finishing our project in the future.
Igem Team Brno, Czech Republic 2021

