IMPLEMENTATION
Blood Type Conversion Kit Design
To bring our project to life, we devised a two-step blood type conversion kit to 1) mediate the enzymatic conversion process, and 2) purify converted RBCs in preparation for transfusion (Fig 1). We plan to pass washed RBCs through a series of airtight columns with bead-immobilized enzymes, where enzymatic conversion will take place. Then, we will isolate cleaved RBCs through centrifugation and filtration with leukodepletion filters.

Figure 1 - Schematic overview of our blood type conversion kit design. In Step 1, washed blood is passed through a column with enzymes immobilized on nickel sepharose beads. In Step 2, cleaved and uncleaved RBCs are separated by through a filtration mechanism in a centrifuge tube.
Step 1: Enzymatic Conversion
Prior to enzymatic conversion, donated whole blood will be washed in 0.9% NaCl to remove the plasma and buffer layer coat, a process that has already been implemented into existing procedures at blood donation/processing centers (Souid, 2008). A previous study has utilized centrifugation settings of 3500 rpm for 5 minutes to isolate RBCs from the solution (Long, 2009).
Mr. Liu, the director for planning at the Taiwan Blood Services Foundation, said that mechanization of our conversion process is important to minimize human labor. He also noted the importance of airtight transfer and sterility. Dr. Kang, a hematologist, proposed converting 1 unit of blood at a time to avoid mixing blood from different donors. We took these considerations into account in the design of our prototype kit.
Fig 2 shows the components involved in our blood type conversion process. We chose to immobilize our enzymes on Nickel sepharose beads (see 1, Fig 2) to minimize the chance of enzymes falling into solution, but maximize the surface area of enzyme and RBC contact. Using Nickel sepharose beads streamlines the construction process of our kit, as enzymes will already be immobilized on these beads for affinity chromatography during protein purification.
We received feedback on this idea from biomedical Legislator Chuang, who expressed concerns over the adverse health effects of beads being accidently transfused into patients. Therefore, we added leukocyte reduction filters (see 2, Fig 2) that can filter RBCs, but trap the nickel sepharose beads (Mizuno, 2013). These filters are currently implemented in many blood processing procedures.
We plan to attach the leukodepletion filter in columns, where they will sit on a layer of biocompatible mesh provided for structural support(see 3, Fig 2). Prior to usage, beads with the appropriate immobilized enzymes will be added into the columns. These columns will be connected in a series of 3, and blood will be passed from the blood bag to the columns with PVC biocompatible tubing (see 4, Fig 2), then through the columns with pressurized flow at a constant rate; the flow speed will thus be faster and more controllable than gravimetric flow. This device allows for hands-off conversion, as enzymes will act on the blood as it travels through the column, limiting human labor of the procedure. The columns are also disposable, which ensures sterility.
We enforced the airtight nature of our device with luer lock connectors (see 5, Fig 2), a standard procedure that will allow us to securely connect the columns together. After blood has been passed through all the columns, it will be collected in a collection centrifuge tube. A special narrow open cap (see 6, Fig 2) was designed to allow blood to flow directly from the columns into the collection tube while still being airtight. After processed blood has been collected into the tube, it can be detached from the columns, closed, and entered into the post-conversion processing step.
Having several columns connected in series provides the added benefit of modularity (Fig 3). Depending on the blood type that we want to convert, we are able to assemble columns with the appropriate enzymes. We thus have 3 different configurations of columns, for type A, type B, and type AB blood.
Our device is optimized to convert 1 unit of blood. For specific dimensions and elaborated details, see
Prototype Hardware and Enzyme Kinetics Model.
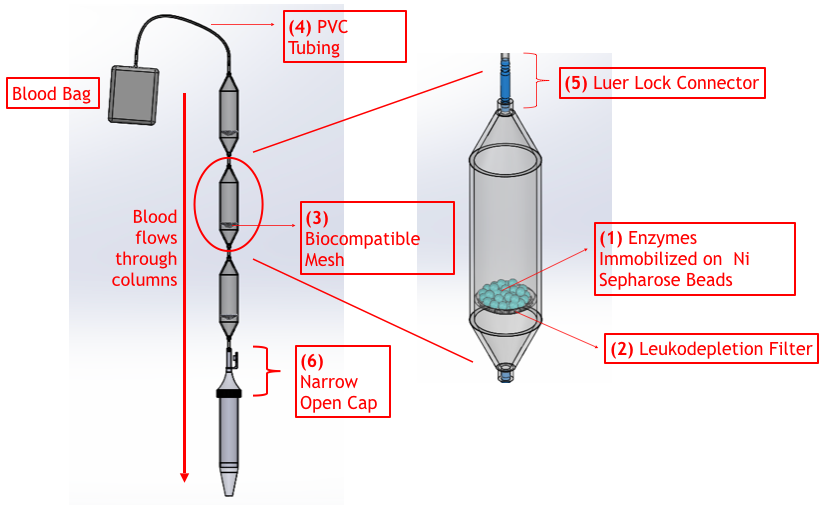
Figure 2 - Labelled Computer Aided Design of Components Involved in Proposed Enzymatic Conversion Process. Blood from a blood bag will be passed through columns, where enzymatic conversion will take place, and be collected in a collection tube. The entire process is airtight.

Figure 3 - Modularity of enzymatic conversion kit. Columns can be assembled in 3 configurations according to blood type.
Step 2: Post-Conversion Purification

Figure 4 - Illustration of post-conversion purification process. Through centrifugation and filtration, cleaved RBCs can be purified from a solution of free/removed antigens, enzymes, and uncleaved RBCs.
Dr. Hsiao, a hematologist, emphasized the importance of eliminating free antigens, and any incompatible/uncleaved red blood cells, from the final product to ensure that the transfusion of processed blood does not result in agglutination in the patients’ bloodstream. Thus, we devised a series of post-conversion purification steps to ensure the safety of the blood type conversion kit (Fig 4).
First, we introduced a centrifugation and washing step, which will allow us to separate RBCs from impurities in solution, such as residual antigens and enzymes from the previous conversion step. Following centrifugation, the supernatant containing the impurities can be decanted and then replaced with a clean saline solution.
We plan to use antibody-antigen agglutination mechanisms to separate cleaved and uncleaved RBCs. The appropriate antibody will be added to the washed solution. We modeled the amount of antibody needed for a given amount of blood in our Antibody-Antigen model. Uncleaved RBCs will react with the antibody and agglutinate, while cleaved RBCs will not. Because agglutinated clumps will be larger in size than individual RBCs, they can be filtered out using a leukodepletion filter. The final solution will thus only contain cleaved RBCs safe for transfusion.
Execution
After speaking to Dr. Liu, the director of planning for the Taiwan Blood Services Foundation, we decided that our project would best be implemented in the processing step of the blood supply chain (Fig 5). We envision our blood type conversion taking place following blood processing, where individual components are isolated and tested for diseases, and before it is stored in the reservoir of the blood processing center (Yang & Lee, 2017).

Figure 5 - Schematic of Blood Supply Chain. We envision our project being implemented after blood processing and before blood packaging and storage.
We wanted to survey our target audience of blood banks and processing centers to determine how to best implement our blood type conversion kit. We received a response from Australian Red Cross Lifeblood. We were informed that their facilities are equipped with centrifuges, and therefore, our post-conversion purification process should be viable. We were also told that a 1 hour conversion time can be feasibly incorporated into their workflow.
Thus, we set the processing time for our kit at 60 minutes, and used experimental data to calculate the amount of enzyme needed (Fig 6). Pre- and Post- conversion steps can take place at 4 degrees centigrade, while enzyme reactionsf will take place at room temperature to increase enzyme efficiency. We determined from percent hemolysis tests that no significant hemolysis results from performing conversion at room temperature for up to 70 minutes.

Figure 6 - Time and temperature breakdown of blood type conversion kit.
Based on our experimental data and model calculations, we determined the volume of enzyme-immobilized beads and antibodies needed to convert 250 mL of blood. Using this information, we compiled the contents that will be included in 1 unit of our “kit” (that converts 1 unit of RBCs). The kit contains all the supplies needed to achieve successful blood type conversion.
Table 1 - Consents Included in Blood Type Conversion Kit

Marketing
Though our project’s blood conversion kit is still in its prototype stage, we wanted to see how it can be used on the current market. Thus, we developed a marketing plan which can be accessed here.
In our Marketing Plan, we expressed the need for our project’s blood converting device and the lack thereof of a similar medical device on the market. We compared UniversO’s processed and converted blood to other types of blood used in blood transfusions, such as regular blood, artificial blood, as well as converted blood, all of which are defined in our marketing plan. Because of our device’s easy integration into the processing of blood for transfusions, efficiency in converting blood types, as well as the need for such a product, we believe that our device will be competitive in the market.
Our target market for our blood conversion product is the United States. The United States has recently declared a nationwide blood shortage, creating a need for our blood conversion device.
The U.S. Food and Drug Administration’s Center of Devices and Radiological Health is in charge of approving and regulating many medical devices in the United States.The availability of resources to approve our blood conversion device will allow our product to be applied in medical settings.
We decided to price our UniversO blood conversion kit at $250USD. We discussed the reasoning for this pricing in our marketing plan.
To create our blood converting device, we plan on partnering with ACHB Enterprise Company, a medical device manufacturing company established in Taiwan, that also has bases in Japan and the US. ACHB has been successful in engineering and manufacturing life science devices. By working with a local-based company that has established divisions in the US, our team can collaborate closely with ACHB to create the most effective product to then be administered in the US.
References
Long, Cassandra, Hidetaka Hara, Zachary Pawlikowski, Naoko Koike, Thomas d’Arville, Peter Yeh, Mohamed Ezzelarab, David Ayares, Mark Yazer, and David K. C. Cooper. 2009.
“Genetically Engineered Pig Red Blood Cells for Clinical Transfusion: Initial in Vitro Studies.” Transfusion 49 (11): 2418–29. https://doi.org/10.1111/j.1537-2995.2009.02306.x.
Mizuno, Ju. 2013. “Use of Microaggregate Blood Filters Instead of Leukocyte Reduction Filters to Purify Salvaged, Autologous Blood for Re-Transfusion during Obstetric Surgery.” Journal of Anesthesia 27 (4): 645–46. https://doi.org/10.1007/s00540-013-1579-7.
“Utilizing Blood Bank Resources/Transfusion Reactions and Complications - ScienceDirect.” n.d. Accessed October 21, 2021. https://www.sciencedirect.com/science/article/pii/B9781416000877501355.
康健. n.d. “「捐血一袋」多少關卡才能到輸血者的手裡? - 康健雜誌.” 康健. Accessed October 21, 2021. https://www.commonhealth.com.tw/article/74358.

