| − | <!-- # TODO: #6 Fix table caption font--><!-- # TODO: #7 Fix citations links font size--><html lang="en"><head><meta charset="utf-8"/><meta content="width=device-width,initial-scale=1" name="viewport"/><title>Results | iGEM Stockholm</title><script src="https://2020.igem.org/common/MathJax-2.5-latest/MathJax.js?config=TeX-AMS-MML_HTMLorMML"></script><link href="https://2021.igem.org/Template:Stockholm/css/contentCSS?action=raw&ctype=text/css" rel="stylesheet"/></head><body><!-- # TODO: #6 Fix table caption font--><!-- # TODO: #7 Fix citations links font size--><nav class="navbar navbar-expand-xl fixed-top"><div class="container d-flex justify-content-between"><a class="navbar-brand d-lg-inline-block" href="https://2021.igem.org/Team:Stockholm"></a><button aria-controls="navbarNav" aria-expanded="false" aria-label="Toggle navigation" class="navbar-toggler" data-target="#navbarNav" data-toggle="collapse" type="button"><span class="navbar-toggler-icon"></span></button><div class="collapse navbar-collapse" id="navbarNav"><ul class="navbar-nav ml-auto"><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarTeamDropdown" role="button">Team</a><div aria-labelledby="navbarTeamDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Team">Team</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Attributions">Attributions</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Collaborations">Collaborations</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Inclusivity">Inclusivity</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Sponsors">Sponsors</a></div></li><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarProjectDropdown" role="button">Project</a><div aria-labelledby="navbarProjectDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Contribution">Contribution</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Description">Description</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Design">Design</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Engineering">Engineering</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Experiments">Experiments</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Notebook">Notebook</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Partnership">Partnership</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Proof_Of_Concept">Proof Of Concept</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Results">Results</a></div></li><li class="nav-item"><a class="nav-link" href="https://2021.igem.org/Team:Stockholm/Model">Model</a></li><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarHuman PracticeDropdown" role="button">Human Practice</a><div aria-labelledby="navbarHuman PracticeDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Human_Practices">Human Practices</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Implementation">Implementation</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Entrepreneurship">Entrepreneurship</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Communication">Communication</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Education">Education</a></div></li><li class="nav-item"><a class="nav-link" href="https://2021.igem.org/Team:Stockholm/Safety">Safety</a></li></ul></div><div class="d-flex" id="themeSwitchWrapper"><i class="far fa-sun"></i><div id="themeSwitch"><label class="switch" for="themeSwitchInput"><input id="themeSwitchInput" type="checkbox"/><span class="slider round"></span></label></div><i class="far fa-moon"></i></div></div></nav><header class="d-flex justify-content-center align-items-center"><div class="container"><h1>Results</h1><p class="lead pl-1">What did we find?</p><hr class="my-4"/></div></header><main><div class="container"><div class="row"><div class="sidebar col-lg-3"><div class="nav" id="contents"><h5>Contents</h5><ul></ul></div></div><div class="content col-lg-9"><article><h1>PCDA Synthesis and Polymerisation</h1><p>Our detection method is based on PCDA polymer vesicles that are able to change colour when subjected to various kinds of stress, such as chemical, mechanical, heat and radiation.</p><p>The first step was to synthesise PCDA polymers from crystalline PCDA monomers. In their polymer form, PCDAs adopt vesicle formations in a water solution. This solution can then be stored at 4 ℃ for extended periods of time.</p><p>We tested the PCDA solution for reactivity to heat and UV-radiation. For heat, a few mL of the solution was poured into a glass test tube and subjected to a burner flame. After a few seconds, the solution turned to a bright orange, indicating a structural change. When it comes to UV-radiation, the colour change is a lot more subtle, changing to a deep purple when left overnight under UV-radiation. This is probably due to the UV lamps’ supplied energy being relatively low, especially compared to the burners energy flux into the solution.</p><div class="image"><img alt="Colour changes showing a structural change" src="https://static.igem.org/mediawiki/2021/d/d5/T--Stockholm--img--Results1.jpg" style="width: 100%"/><p>Figure 1: Colour changes showing a structural change</p></div><p>Spectrophotometric measurements of the colour change were performed and the absorbance maxima changes from around 640 nm to around 500 nm. Both peaks visible on the spectrum are shifted to lower wavelengths when the PCDA solution is heated.</p><div class="image"><img alt="Spectrometric absorbances" src="https://static.igem.org/mediawiki/2021/f/f5/T--Stockholm--img--Results2.jpg" style="width: 100%"/><p>Figure 2: Spectrometric absorbances</p></div><h1>PCDA - Protein A Aptamer Conjugation</h1><p>The next step was to conjugate our PCDA with the aptamer and then test it using protein A. After incubation of the protein A with the PCDA-aptamer conjugate we did not detect any color change so we tested if the protein A aptamer is actually binding protein A. We examined that in 3 different ways:</p><ul><li>Protein A coated beads</li><li>Chromatography using a protein A coated column</li><li>Binding assay using a fluorescent protein A aptamer</li></ul><p>Unfortunately, the first two ways, beads and chromatography, yielded no positive results (images 3,4). However, the binding assay kinda worked.</p><p>3 IMAGES HERE</p><h1>SELEX</h1><p>Another crucial part of our project was to create the two aptamers, one for the lipoteichoic acid and one for c. acnes, using SELEX. We faced a lot of problems during this process. The main obstacle was the optimization of the PCR. From wrong concentrations of PCR reagents to contaminations and wrong primers we spent a lot of time troubleshooting in order to make the PCR work since it is the most important part of the SELEX process. Everything is written in detail in the engineering and in our troubleshooting guide.</p><p>IMAGE HERE</p><p>In our last days in the lab we got positive results for our first round of SELEX, which means that, since SELEX is a repetitive process, not only our protocol works but also if we had more time we would probably be able to have successful rounds of SELEX.</p><h1>Cloning</h1><h1>Headings</h1><h2>Level 2 Heading</h2><h3>Level 3 Heading</h3><h4>Level 4 Heading</h4><h1>Emphasis</h1><p>This is regular text.</p><p><strong>This is bold text.</strong> <strong>This is also bold text.</strong></p><p><em>This is italic text.</em> _This is also italix text.*</p><p><em><strong>This text is bold and italic both.</strong></em> <em><strong>This text is bold and italic both.</strong></em></p><p><s>Strikethrough</s></p><h1>Lists</h1><ol><li>This is item one.</li><li>This is item two.</li><li>It's okay to number every item as 1.<ul><li>This is a nested list.</li><li>Use asterisk for an unordered list,</li><li>Further nesting?</li></ul><ul><li>Plus sign also works.</li></ul><ul><li>And so does minus.</li></ul></li><li><s>Birds aren't real.</s></li></ol><h1>Links</h1><p><a href="https://google.com">Google</a></p><p><a href="https://2021.igem.org/Team:Stockholm/Results/Link-goes-in-parenthesis">Text goes in square brackets</a></p><h1>Blockquote</h1><blockquote><p>This is a blockquote. This continues in the same paragraph.</p><p>To change the paragraph, you have to leave a line.</p></blockquote><blockquote><p>This is another blockquote.</p><p><strong>Pranav Ballaney, 2020</strong></p></blockquote><h1>Images</h1><p>This is a regular paragraph.</p><h4>This is a level 4 heading.</h4><p><a href="google.com">This is a link</a></p><div class="image"><img alt="Caption" src="https://static.igem.org/mediawiki/2021/9/92/T--Stockholm--img--Description--josh-withers.jpg" style="width: 100%"/><p>Figure 3: Caption</p></div><p>Back to regular text.</p><h1>Tables</h1><p>This is a regular paragraph, which precedes the table. in fact, i wanna do math here, here is mt math $$i\hbar\gamma^\mu\partial_\mu\psi = mc\psi$$ what good math When you want to insert a table, indent one level back and specify the plugin. Then indent inside again and start writing the table.</p><table><caption id="table1captiongoeshere">Table 1: Caption goes here.</caption><thead><tr><th>This is</th><th>the table</th><th>header row</th></tr></thead><tbody><tr><td>1</td><td>2</td><td>3</td></tr><tr><td>4</td><td>5</td><td>6</td></tr></tbody></table><p>And when you're done, go back to the regular markdown filter.</p><p><strong>Example 2:</strong></p><table><thead><tr><th style="text-align:left">Left aligned</th><th style="text-align:center">Center aligned</th><th style="text-align:right">Right aligned</th></tr></thead><tbody><tr><td style="text-align:left">1</td><td style="text-align:center">2</td><td style="text-align:right">3</td></tr><tr><td style="text-align:left">4</td><td style="text-align:center">5</td><td style="text-align:right">6</td></tr></tbody></table><p><strong>Example 3:</strong></p><table><caption id="table3captiongoeshere">Table 3: Caption goes here.</caption><thead><tr><th></th><th colspan="2" style="text-align:center">Grouping</th></tr><tr><th>First Header</th><th style="text-align:center">Second Header</th><th style="text-align:right">Third Header</th></tr></thead><tbody><tr><td>Content</td><td colspan="2" style="text-align:center"><em>Long Cell</em></td></tr><tr><td>Content</td><td style="text-align:center"><strong>Cell</strong></td><td style="text-align:right">Cell</td></tr><tr><td>New section</td><td style="text-align:center">More</td><td style="text-align:right"><a href="https://google.com">Data</a></td></tr><tr><td>And more</td><td colspan="2" style="text-align:center">With an escaped '|'</td></tr></tbody></table><h1>Definitions</h1><p><dfn>Term ~ Definition</dfn></p><p>This can come <dfn>anywhere in ~ the text</dfn>.</p><h1>MathJax</h1><p>$$\int_{0}^{\infty} e^{-x^2} dx = \frac{\sqrt{\pi}}{2}$$</p><p>$$-\frac{\hbar^2}{2m}\nabla^2\psi + V\psi = E\psi$$</p><p>$$i\hbar\gamma^\mu\partial_\mu\psi = mc\psi$$</p><p>$$y_k = \frac{1}{\sqrt{N}}\sum_{n=0}^{N-1}x_n\omega_N^{kn}$$</p><h1>Citations</h1><p>In text citation for a research article with a DOI. <a href="#citation2">Rosano et al., 2019</a></p><p>In text citation for another research article with a DOI. <a href="#citation1">Allen & Sheridan, 2015</a></p><p>In text citation for a book with no DOI. <a href="#citation3">Ingalls, 2013</a></p><p>In text citation for a website with institutional author. <a href="#citation4">TNAU Agritech Portal, n.d.</a></p><p>In text citation for a website with an author. <a href="#citation5">Author, n.d.</a></p></article><article id="references"><h1>References</h1><ol><li id="citation1"><p class="author">Allen, M. J., & Sheridan, S. C. (2015).</p><cite>Mortality risks during extreme temperature events (ETEs) using a distributed lag non-linear model.</cite><p><span class="journalTitle">International Journal of Biometeorology</span> <span class="journalInfo">62(1), 57-67.</span></p><a class="in-text" href="https://doi.org/10.1007/s00484-015-1117-4" rel="noopener" target="_blank">CrossRef</a><a class="in-text" href="https://scholar.google.com/scholar?q=Mortality risks during extreme temperature events (ETEs) using a distributed lag non-linear model." rel="noopener" target="_blank">Google Scholar</a><a class="in-text" href="#intext1">Back to text</a></li><li id="citation2"><p class="author">Rosano, A., Bella, A., Gesualdo, F., Acampora, A., Pezzotti, P., Marchetti, S., ... & Rizzo, C. (2019).</p><cite>Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14-2016/17 seasons).</cite><p><span class="journalTitle">International Journal of Infectious Diseases</span> <span class="journalInfo">88, 127-134.</span></p><a class="in-text" href="https://doi.org/10.1016/j.ijid.2019.08.003" rel="noopener" target="_blank">CrossRef</a><a class="in-text" href="https://scholar.google.com/scholar?q=Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14-2016/17 seasons)." rel="noopener" target="_blank">Google Scholar</a><a class="in-text" href="#intext2">Back to text</a></li><li id="citation3"><p class="author">Ingalls, B. P. (2013).</p><cite>Mathematical modeling in systems biology: An introduction.</cite><p><span class="details">MIT Press.</span></p><a class="in-text" href="https://books.google.co.in/books?id=OYr6AQAAQBAJ" rel="noopener" target="_blank">Google Books</a><a class="in-text" href="#intext3">Back to text</a></li><li id="citation4"><p class="author"></p><cite>Agriculture: Crop production: Sugarcane. TNAU Agritech Portal.</cite><p><span class="details">(March 15, 2019). Retrieved on June 22, 2020. from </span><a class="in-text" href="https://google.com" rel="noopener" target="_blank">https://google.com</a></p><a class="in-text" href="#intext4">Back to text</a></li><li id="citation5"><p class="author">Author Name. (n.d.).</p><cite>Agriculture: Crop production: Sugarcane. TNAU Agritech Portal.</cite><p><span class="details">Retrieved on June 22, 2020. from </span><a class="in-text" href="https://google.com" rel="noopener" target="_blank">https://google.com</a></p><a class="in-text" href="#intext5">Back to text</a></li></ol></article></div></div></div></main><footer><div class="container"><a class="fafa" href="https://www.facebook.com/igemstockholm/" target="_blank"><i class="fab fa-facebook" style="font-size:60px;"></i></a><a class="fafa" href="https://www.instagram.com/igemstockholm" target="_blank"><i class="fab fa-instagram" style="font-size:60px;"></i></a><a class="fafa" href="https://www.linkedin.com/company/igemstockholm" target="_blank"><i class="fab fa-linkedin" style="font-size:60px;"></i></a><a class="fafa" href="https://www.youtube.com/channel/UCh_a6JvWdh6N_i5tYFFcpyw" target="_blank"><i class="fab fa-youtube" style="font-size:60px;"></i></a><a class="fafa" href="mailto: igem.sthlm@gmail.com" target="_blank"><i class="fas fa-envelope" style="font-size:60px;"></i></a></div><br/><div class="container"><a class="uni-logo" href="https://ki.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/b/bb/T--Stockholm--img--ki-whiteback-modified.png" style="width:100px;height:100px;"/></a><a class="uni-logo" href="https://kth.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/e/e2/T--Stockholm--img--kthwhite-modified.png" style="width:100px;height:100px;"/></a><a class="uni-logo" href="https://su.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/d/da/T--Stockholm--img--stockholmuni-modified.png" style="width:100px;height:100px;"/></a></div><br/><div class="container"><p>Sample template built using the iGEM Wiki Starter Pack by BITS Goa.</p><p>Code released under the MIT license.</p><p>Based on <a href="https://getbootstrap.com">Bootstrap</a> and themes <a href="https://bootswatch.com/flatly/">Flatly</a> and <a href="https://bootswatch.com/darkly/">Darkly</a> from <a href="https://bootswatch.com/">Bootswatch</a>.</p><p>Some content from the <a href="https://2020.igem.org/Team:Example">iGEM Example Wiki</a>. Images from <a href="https://unsplash.com">Unsplash</a>. Web fonts from <a href="https://fonts.google.com">Google</a>.</p></div></footer><script src="https://2021.igem.org/Template:Stockholm/content-bundleJS?action=raw&ctype=text/javascript"></script></body></html>
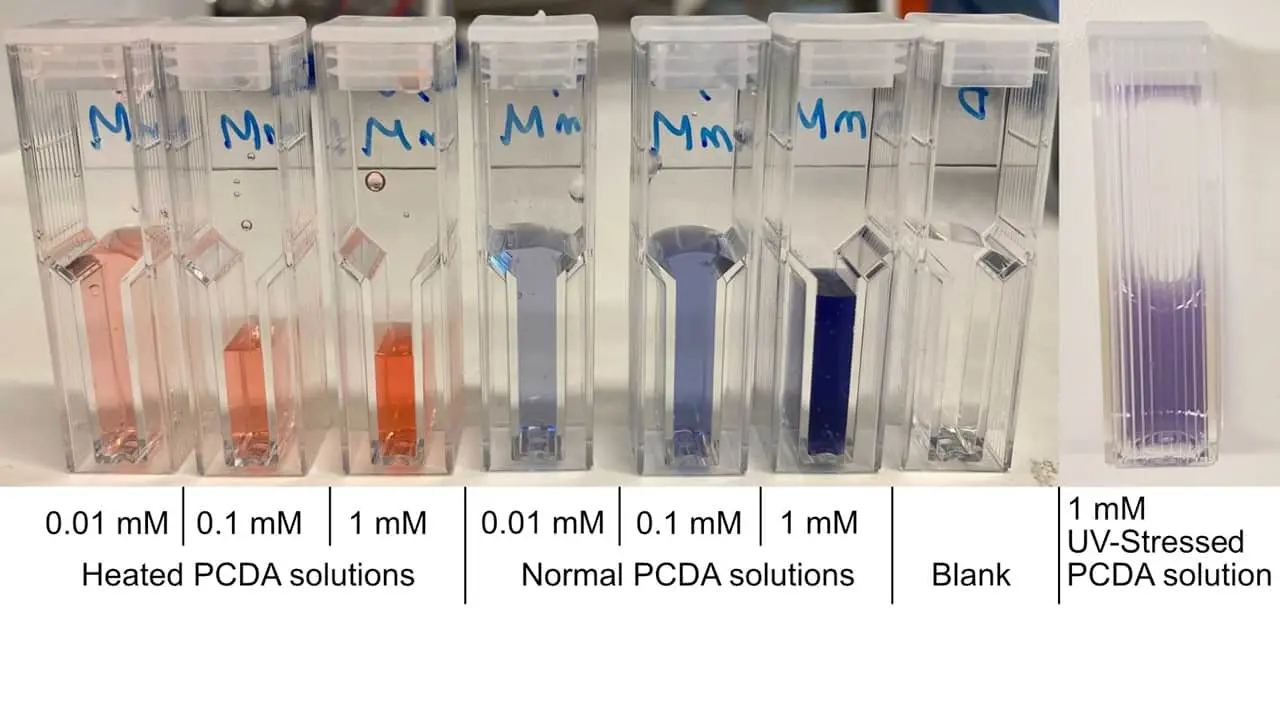
| + | <!-- # TODO: #6 Fix table caption font--><!-- # TODO: #7 Fix citations links font size--><html lang="en"><head><meta charset="utf-8"/><meta content="width=device-width,initial-scale=1" name="viewport"/><title>Results | iGEM Stockholm</title><script src="https://2020.igem.org/common/MathJax-2.5-latest/MathJax.js?config=TeX-AMS-MML_HTMLorMML"></script><link href="https://2021.igem.org/Template:Stockholm/css/contentCSS?action=raw&ctype=text/css" rel="stylesheet"/></head><body><!-- # TODO: #6 Fix table caption font--><!-- # TODO: #7 Fix citations links font size--><nav class="navbar navbar-expand-xl fixed-top"><div class="container d-flex justify-content-between"><a class="navbar-brand d-lg-inline-block" href="https://2021.igem.org/Team:Stockholm"></a><button aria-controls="navbarNav" aria-expanded="false" aria-label="Toggle navigation" class="navbar-toggler" data-target="#navbarNav" data-toggle="collapse" type="button"><span class="navbar-toggler-icon"></span></button><div class="collapse navbar-collapse" id="navbarNav"><ul class="navbar-nav ml-auto"><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarTeamDropdown" role="button">Team</a><div aria-labelledby="navbarTeamDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Team">Team</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Attributions">Attributions</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Collaborations">Collaborations</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Inclusivity">Inclusivity</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Sponsors">Sponsors</a></div></li><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarProjectDropdown" role="button">Project</a><div aria-labelledby="navbarProjectDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Contribution">Contribution</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Description">Description</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Design">Design</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Engineering">Engineering</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Experiments">Experiments</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Notebook">Notebook</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Partnership">Partnership</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Proof_Of_Concept">Proof Of Concept</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Results">Results</a></div></li><li class="nav-item"><a class="nav-link" href="https://2021.igem.org/Team:Stockholm/Model">Model</a></li><li class="nav-item dropdown"><a aria-expanded="false" aria-haspopup="true" class="nav-link dropdown-toggle" data-toggle="dropdown" href="#" id="navbarHuman PracticeDropdown" role="button">Human Practice</a><div aria-labelledby="navbarHuman PracticeDropdown" class="dropdown-menu"><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Human_Practices">Human Practices</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Implementation">Implementation</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Entrepreneurship">Entrepreneurship</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Communication">Communication</a><a class="dropdown-item" href="https://2021.igem.org/Team:Stockholm/Education">Education</a></div></li><li class="nav-item"><a class="nav-link" href="https://2021.igem.org/Team:Stockholm/Safety">Safety</a></li></ul></div><div class="d-flex" id="themeSwitchWrapper"><i class="far fa-sun"></i><div id="themeSwitch"><label class="switch" for="themeSwitchInput"><input id="themeSwitchInput" type="checkbox"/><span class="slider round"></span></label></div><i class="far fa-moon"></i></div></div></nav><header class="d-flex justify-content-center align-items-center"><div class="container"><h1>Results</h1><p class="lead pl-1">What did we find?</p><hr class="my-4"/></div></header><main><div class="container"><div class="row"><div class="sidebar col-lg-3"><div class="nav" id="contents"><h5>Contents</h5><ul></ul></div></div><div class="content col-lg-9"><article><h1>PCDA Synthesis and Polymerisation</h1><p>Our detection method is based on the 10,12-Pentacosadiynoic acid (PCDA) polymer vesicles which can change colour when subjected to various kinds of stress, such as chemical, mechanical, heat and radiation.</p><p>The first step was to synthesise PCDA polymers from crystalline PCDA monomers. In their polymer form, PCDAs adopt vesicle formations in a water solution. This solution can then be stored at 4 degrees Celsius for extended periods of time.</p><p>We tested the PCDA solution for reactivity to heat and UV-radiation. The results of this test are shwon in Figure 1. For heat, a few mL of the solution was poured into a glass test tube and subjected to a burner flame. After a few seconds, the solution turned to a bright orange, indicating a structural change. When it comes to UV-radiation, the colour change is a lot more subtle, changing to a deep purple when left overnight under UV-radiation. This is probably due to the UV lamps' supplied energy being relatively low, especially compared to the burner's energy flux into the solution.</p><div class="image"><img alt="PCDA solutions subjected to different stress conditions (heat, UV radiation, normal), and subsequently diluted for spectrophotometric measurements. " src="https://static.igem.org/mediawiki/2021/d/d5/T--Stockholm--img--Results1.jpg" style="width: 100%"/><p>Figure 1: PCDA solutions subjected to different stress conditions (heat, UV radiation, normal), and subsequently diluted for spectrophotometric measurements.</p></div><p>Spectrophotometric measurements of the colour change were performed with the solutions from Figure 1, and the results are shown in Figure 2. The absorbance maxima changes from around 640 nm to around 500 nm. Both peaks visible on the spectrum are shifted to lower wavelengths when the PCDA solution is heated.</p><div class="image"><img alt="Absorbance spectrums, shown with different coloured curves of A: Blue PCDA at 1 mM (Black), 0,1 mM (Red) and 0,01 mm (Blue); B: Red PCDA at 1 mM (Blue), 0,01 mM (Purple), 0,01 mM (Red) and 0,001 mM (Black)." src="https://static.igem.org/mediawiki/2021/f/f5/T--Stockholm--img--Results2.jpg" style="width: 100%"/><p>Figure 2: Absorbance spectrums, shown with different coloured curves of A: Blue PCDA at 1 mM (Black), 0,1 mM (Red) and 0,01 mm (Blue); B: Red PCDA at 1 mM (Blue), 0,01 mM (Purple), 0,01 mM (Red) and 0,001 mM (Black).</p></div><h1>PCDA - Protein A Aptamer Conjugation</h1><p>The next step was to conjugate our PCDA with the aptamer and then test it using protein A. After incubation of the protein A with the PCDA-aptamer conjugate we did not detect any colour change so we needed to test if the protein A aptamer is actually binding protein A. We investigated the protein A aptamer binding affinity with three different experiments: first, using protein A-coated beads, then with chromatography, and finally by performing a binding assay.</p><h2>Protein A coated beads</h2><p>Early on, after noticing that the protein A aptamer might not bind to its target, we used protein A coated beads for testing the binding qualitatively. After following the protocol provided with the beads, we ran the fraction from elution in an agarose gel, which is shown in Figure 3. After elution, the one sample of the fraction was desalted, in order to have a less harsh environment for the aptamers. However, as can be seen on the gel, the wells appear empty, which means that the aptamers most likely did not bind to protein A. It is nonetheless possible for the binding to be too weak, resulting in very little aptamers being eluted and thus not showing up on the gel.</p><div class="image"><img alt="Agarose gel with the protein A beads products. In the last column had the product after the elution of the beads where the eluted aptamers are supposed to be. The second-to-last column had the eluted product which was also subsequently desalted." src="https://static.igem.org/mediawiki/2021/9/9c/T--Stockholm--img--Results_3.png" style="width: 100%"/><p>Figure 3: Agarose gel with the protein A beads products. In the last column had the product after the elution of the beads where the eluted aptamers are supposed to be. The second-to-last column had the eluted product which was also subsequently desalted.</p></div><h2>Chromatography using a protein A coated column</h2><p>Since the test using the magnetic beads could have a too low concentration in the elution for it to be detectable on a gel, we set up a chromatography experiment with a protein A column. The resulting chromatogram is shown in Figure 4. Fractions from the sample application run-through and from the elution were analysed using an agarose gel, which is also shown in figure 4. The wells containing the sample application run-through had a lot of contents, while the fractions from the elution were empty. This again shows that the protein A aptamer was not binding to protein A.</p><div class="image"><img alt="Chromatography results. The chromatogram is shown at the top. Tubes 1 to 4 are from sample application while tubes from 15 to 24 are fractions from elution. The agarose gel with the chromatography products is shown at the bottom. Each well is labelled with the corresponding tube from the chromatography. From left to right, the first well has ladder, the next four have samples from tubes 1-4 and the last ten have samples from tubes 15-24." src="https://static.igem.org/mediawiki/2021/f/fe/T--Stockholm--img--Results_4.png" style="width: 100%"/><p>Figure 4: Chromatography results. The chromatogram is shown at the top. Tubes 1 to 4 are from sample application while tubes from 15 to 24 are fractions from elution. The agarose gel with the chromatography products is shown at the bottom. Each well is labelled with the corresponding tube from the chromatography. From left to right, the first well has ladder, the next four have samples from tubes 1-4 and the last ten have samples from tubes 15-24.</p></div><h2>Binding assay using a fluorescent protein A aptamer</h2><p>The previous results suggest that our protein A aptamer is not binding protein A as expected. In order to finally figure out the aptamer affinity issues we designed the following experiment using protein A aptamer labelled with the fluorophore fluorescein (5' 6-FAM aptamer).</p><p>We incubated 100pmol, 200pmol and 300pmol of fluorescent aptamer in triplicates in a 96-well plate coated with protein A for 45 minutes. Then, washed the wells with PBS to remove unbound aptamer molecules and measured the fluorescence intensity in a plate reader.</p><p>To infer the pmol of aptamer that had successfully bound to protein A, we created the following standard curve using known pmol quantities of 5' 6-FAM aptamer.</p><div class="image"><img alt="5' 6-FAM aptamer standard curve showing the relationship between RFUs (Relative fluorescence units) and aptamer amount (pmol). A linear regression model was used to determine the line, regression equation and R^2 coefficient." src="https://static.igem.org/mediawiki/2021/c/cf/T--Stockholm--img--results_5.JPG" style="width: 100%"/><p>Figure 5: 5' 6-FAM aptamer standard curve showing the relationship between RFUs (Relative fluorescence units) and aptamer amount (pmol). A linear regression model was used to determine the line, regression equation and R^2 coefficient.</p></div><p>Using the regression equation, we inferred the pmol of aptamer that bound to the protein A.</p><p>The total percentage of bound aptamer was then calculated for each sample by dividing the pmol of aptamer bound by total pmol aptamer incubated.</p><table><thead><tr><th>Amount [pmol]</th><th>RFU</th><th>Inferred amount bound aptamer [pmol]</th><th>Percentage bound aptamer</th></tr></thead><tbody><tr><td rowspan="3">100</td><td>62</td><td>0.527</td><td>0.527</td></tr><tr><td>62</td><td>0.527</td><td>0.527</td></tr><tr><td>51</td><td>0.434</td><td>0.434</td></tr><tr><td rowspan="3">200</td><td>92</td><td>0.782</td><td>0.391</td></tr><tr><td>171</td><td>1.45</td><td>0.727</td></tr><tr><td>93</td><td>0.791</td><td>0.395</td></tr><tr><td rowspan="3">300</td><td>137</td><td>1.16</td><td>0.388</td></tr><tr><td>114</td><td>0.969</td><td>0.323</td></tr><tr><td>63</td><td>0.536</td><td>0.179</td></tr></tbody></table><p><em>Table 1. Amount of aptamer bound (pmol) and percentage of bound aptamer determined by the binding affinity assay.</em></p><p>Based on these results, we concluded that the protein A aptamer we chose for our proof of concept does not have sufficient binding affinity to its target.</p><p>This conclusion is also supported by protein A - aptamer docking model (link to model page), where no evidence of obvious strong interactions was found.</p><h1>SELEX</h1><p>Another crucial part of our project was to create the two aptamers, one for lipoteichoic acid and one for C. acnes, using SELEX. We faced a lot of problems during this process. The main obstacle was the optimization of the PCR. From wrong concentrations of PCR reagents to contaminations and wrong primers we spent a lot of time troubleshooting in order to make the PCR work since it is the most important part of the SELEX process. One example is shown in figure 6, which displays an agarose gel with PCR troubleshooting products, where two different polymerases, temperatures and primer concentrations were tried. Everything is written in detail in the engineering and in our troubleshooting guide.</p><div class="image"><img alt="Troubleshooting example. PCR products run in a 2% agarose gel. Two different polymerases (Takara Titanium Taq DNA Polymerase and DreamTaq DNA Polymerase), primer concentrations (0.5 mM and 1mM) and annealing temperatures (55°C and 60°C) were tested. Additionally, a non template control (NTC) for each polymerase and annealing temperature was run." src="https://static.igem.org/mediawiki/2021/e/e1/T--Stockholm--img--Results_6.png" style="width: 100%"/><p>Figure 6: Troubleshooting example. PCR products run in a 2% agarose gel. Two different polymerases (Takara Titanium Taq DNA Polymerase and DreamTaq DNA Polymerase), primer concentrations (0.5 mM and 1mM) and annealing temperatures (55°C and 60°C) were tested. Additionally, a non template control (NTC) for each polymerase and annealing temperature was run.</p></div><p>After all the hard work over the summer, we were delighted to get positive SELEX results during our last few days in the lab. We performed a successful first amplification of the libraries in order to figure out the right amount of PCR amplification cycles needed for the second amplification, based on the gel image shown in figure 7. Then the remainder of both libraries were amplified with 45 cycles of PCR in total, since the pre-amplification accounted for 15 cycles and the optimal number of cycles after pre-amplification was chosen to be 30.</p><div class="image"><img alt="Agarose gel showing library amplification PCR products, with LTA SELEX in the left part of the gel and Cell SELEX on the right part of the gel. For each, the wells have samples with an increasing number of PCR amplification cycles, from left to right. The strongest bands with the least amount of laddering were picked for both types of SELEX, and both corresponded to 30 cycles (highlighted with dashed boxes)." src="https://static.igem.org/mediawiki/2021/f/f4/T--Stockholm--img--Results_7.png" style="width: 100%"/><p>Figure 7: Agarose gel showing library amplification PCR products, with LTA SELEX in the left part of the gel and Cell SELEX on the right part of the gel. For each, the wells have samples with an increasing number of PCR amplification cycles, from left to right. The strongest bands with the least amount of laddering were picked for both types of SELEX, and both corresponded to 30 cycles (highlighted with dashed boxes).</p></div><p>The PCR products from the second amplification can be seen in figure 8. Then, the PCR products were pooled for each SELEX type, after which the sense and antisense strands were separated. The sense strands were eluted and desalted, which yielded very little product as can be seen in figure 9, and we are unsure whether it is the right product that was obtained.</p><div class="image"><img alt="2% agarose gel of the amplification products for (A): LTA SELEX and (B): Cell SELEX; after 45 cycles. For each type of SELEX, each well had a sample from a separate PCR tube. The last wells had non-amplified libraries as samples." src="https://static.igem.org/mediawiki/2021/1/1a/T--Stockholm--img--Results_8png.png" style="width: 100%"/><p>Figure 8: 2% agarose gel of the amplification products for (A): LTA SELEX and (B): Cell SELEX; after 45 cycles. For each type of SELEX, each well had a sample from a separate PCR tube. The last wells had non-amplified libraries as samples.</p></div><div class="image"><img alt="Agarose gel of fractions from desalting after the elution of the sense DNA strands. The first well from the left after the ladder had sample from the run-through of the sample, the next well had sample from the eluted fraction, and the second-to-last well had sample from the left-over volume that came after the sample elution." src="https://static.igem.org/mediawiki/2021/e/eb/T--Stockholm--img--Results_9.png" style="width: 100%"/><p>Figure 9: Agarose gel of fractions from desalting after the elution of the sense DNA strands. The first well from the left after the ladder had sample from the run-through of the sample, the next well had sample from the eluted fraction, and the second-to-last well had sample from the left-over volume that came after the sample elution.</p></div><p>As SELEX is a repetitive process, we were able to prove that each part of our protocol works. Given more time, we would have worked towards achieving multiple successful rounds of SELEX.</p><h1>BioBrick Engineering</h1><p>We set out to create a recombinant protein A labelled with the yellow chromoprotein amilGFP as a reporter. In order to do this, we selected using pre-existing parts from the registry: the amilGFP BioBrick (BBa_K1073024), as well as staphylococcal protein A BioBrick (BBa_K103003).</p><p>Following our cloning strategy (Experiments > BioBrick engineering), we first successfully linearised the plasmid containing the amilGFP BioBrick by digesting with the SpeI and PstI restriction enzymes.</p><div class="image"><img alt="Digested and undigested product in 1% agarose gel. Lane C shows the undigested plasmid. Two different bands can be observed, corresponding to two different conformations of the DNA plasmid: open coiled (OC) and Covalently Closed Circle (CCC). Lane D shows the digested plasmid product." src="https://static.igem.org/mediawiki/2021/8/89/T--Stockholm--img--amilGFP-vector-digestion.png" style="width: 100%"/><p>Figure 10: Digested and undigested product in 1% agarose gel. Lane C shows the undigested plasmid. Two different bands can be observed, corresponding to two different conformations of the DNA plasmid: open coiled (OC) and Covalently Closed Circle (CCC). Lane D shows the digested plasmid product.</p></div><p>Once we confirmed the plasmid had been linearised, we isolated it by performing gel extraction.</p><div class="image"><img alt="1% agarose gel with linearised amilGFP plasmid, before (A) and after (B) the gel extraction. (A) AmilGFP plasmid was digested with SpeI and PstI. (B) Using the UV table, the fragment of interest was isolated, leaving out the smear." src="https://static.igem.org/mediawiki/2021/a/ac/T--Stockholm--img--amilGFP-gel-extraction.png" style="width: 100%"/><p>Figure 11: 1% agarose gel with linearised amilGFP plasmid, before (A) and after (B) the gel extraction. (A) AmilGFP plasmid was digested with SpeI and PstI. (B) Using the UV table, the fragment of interest was isolated, leaving out the smear.</p></div><p>Then, we moved on to amplify the protein A BioBrick by PCR, using VF2 and VR standard primers. The PCR product had the expected size: ~ 500 bp.</p><div class="image"><img alt="PCR amplification products on a 2% agarose gel. Lanes 1 to 3 contain the same PCR product. Lane 4 is PCR negative control (non-template control)." src="https://static.igem.org/mediawiki/2021/8/81/T--Stockholm--img--PCR-amplification-protein-A.png" style="width: 100%"/><p>Figure 12: PCR amplification products on a 2% agarose gel. Lanes 1 to 3 contain the same PCR product. Lane 4 is PCR negative control (non-template control).</p></div><p>After performing a PCR clean up, the amplified protein A BioBrick was digested using XbaI and PstI restriction enzymes. The expected fragments were:</p><ul><li>Protein A BioBrick -> ~ 235 bp.</li><li>VF2 - XbaI -> 133 bp</li><li>PstI - VR -> 154 bp</li></ul><p>We could not use PCR clean up to get rid of the digestion leftovers, since it removes oligos up until 40 bp. Therefore, the protein A BioBrick (digestion product) was isolated by gel extraction.</p><div class="image"><img alt="1% agarose gel with digested protein A BioBrick, before (A) and after (B) the gel extraction. (A) The electrophoresis was run until the protein A BioBrick (~235 bp) was separated from other digestion products (~150 bp). (B) Using the UV table, the fragment of interest was isolated successfully." src="https://static.igem.org/mediawiki/2021/e/e6/T--Stockholm--img--Protein-A-gel-digestion.png" style="width: 100%"/><p>Figure 13: 1% agarose gel with digested protein A BioBrick, before (A) and after (B) the gel extraction. (A) The electrophoresis was run until the protein A BioBrick (~235 bp) was separated from other digestion products (~150 bp). (B) Using the UV table, the fragment of interest was isolated successfully.</p></div><p>The next step was to ligate the protein A BioBrick and the vector containing amilGFP plasmid to create the recombinant protein. Compatibility between XbaI - SpeI and PstI - PstI sticky ends allowed for directional cloning of the protein A BioBrick into the amilGFP plasmid. Ligation was performed by incubation of both parts with T4 ligase. The ligation product was then used to transform TOP10 E. coli competent cells.</p><p>Several yellow colonies, indicating amilGFP expression, were selected to set O/N cultures, from which glycerol stocks and minipreps were prepared. Sanger sequencing confirmed our plasmid had the desired sequence.</p><div class="image"><img alt="Yellow E. coli colonies due to amilGFP expression on LB-agar plate." src="https://static.igem.org/mediawiki/2021/5/5b/T--Stockholm--img--Yellow-colonies.png" style="width: 100%"/><p>Figure 14: Yellow E. coli colonies due to amilGFP expression on LB-agar plate.</p></div><p>Ultimately, in order to create a functional recombinant protein, we had to mutate two sequential stop codons at the end of the amilGFP coding sequence by using Quikchange site-directed mutagenesis. During the PCR step, TAA STOP codons were substituted by TCA Serine codons thanks to the primers, which had these modifications incorporated (Figure quikchange). Three different annealing temperatures were used in the PCR: 55 degrees Celsius, 58 degrees Celsius and 60 degrees Celsius.</p><p>Then, DpnI endonuclease digested the non-mutated parental DNA strands, and the final product was transformed into TOP10 E. coli competent cells.</p><div class="image"><img alt="Quikchange Forward and Reverse primers are complementary to the target sequence at the end of the amilGFP CDS (BBa_K1073024), but include the desired point mutations (mismatches) that were incorporated by PCR. Retrieved from SnapGene." src="https://static.igem.org/mediawiki/2021/8/88/T--Stockholm--img--Quikchange-primers-wiki.png" style="width: 100%"/><p>Figure 15: Quikchange Forward and Reverse primers are complementary to the target sequence at the end of the amilGFP CDS (BBa_K1073024), but include the desired point mutations (mismatches) that were incorporated by PCR. Retrieved from SnapGene.</p></div><p>Plates transformed with products from 55 degrees Celsius and 58 degrees Celsius PCR annealing temperatures had colonies. Expression of functional amilGFP could be confirmed with the presence of bright yellow colonies in LB-agar plates and yellow cell pellets in liquid culture.</p><p>Finally, the sequence of the newly engineered amilGFP-proteinA BioBrick (BBa_K4071000) was verified by Sanger sequencing and the part was validated.</p><div class="image"><img alt="Vector map of pSB1C3 containing the final version of the amilGFP-protein A BioBrick (BBa_K4071000)." src="https://static.igem.org/mediawiki/2021/d/d7/T--Stockholm--img--amilGFP-protA-BioBrick-Map.png" style="width: 100%"/><p>Figure 16: Vector map of pSB1C3 containing the final version of the amilGFP-protein A BioBrick (BBa_K4071000).</p></div></article></div></div></div></main><footer><div class="container"><a class="fafa" href="https://www.facebook.com/igemstockholm/" target="_blank"><i class="fab fa-facebook" style="font-size:60px;"></i></a><a class="fafa" href="https://www.instagram.com/igemstockholm" target="_blank"><i class="fab fa-instagram" style="font-size:60px;"></i></a><a class="fafa" href="https://www.linkedin.com/company/igemstockholm" target="_blank"><i class="fab fa-linkedin" style="font-size:60px;"></i></a><a class="fafa" href="https://www.youtube.com/channel/UCh_a6JvWdh6N_i5tYFFcpyw" target="_blank"><i class="fab fa-youtube" style="font-size:60px;"></i></a><a class="fafa" href="mailto: igem.sthlm@gmail.com" target="_blank"><i class="fas fa-envelope" style="font-size:60px;"></i></a></div><br/><div class="container"><a class="uni-logo" href="https://ki.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/b/bb/T--Stockholm--img--ki-whiteback-modified.png" style="width:100px;height:100px;"/></a><a class="uni-logo" href="https://kth.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/e/e2/T--Stockholm--img--kthwhite-modified.png" style="width:100px;height:100px;"/></a><a class="uni-logo" href="https://su.se" target="_blank"><img src="https://static.igem.org/mediawiki/2021/d/da/T--Stockholm--img--stockholmuni-modified.png" style="width:100px;height:100px;"/></a></div><br/><div class="container"><p>Sample template built using the iGEM Wiki Starter Pack by BITS Goa.</p><p>Code released under the MIT license.</p><p>Based on <a href="https://getbootstrap.com">Bootstrap</a> and themes <a href="https://bootswatch.com/flatly/">Flatly</a> and <a href="https://bootswatch.com/darkly/">Darkly</a> from <a href="https://bootswatch.com/">Bootswatch</a>.</p><p>Some content from the <a href="https://2020.igem.org/Team:Example">iGEM Example Wiki</a>. Images from <a href="https://unsplash.com">Unsplash</a>. Web fonts from <a href="https://fonts.google.com">Google</a>.</p></div></footer><script src="https://2021.igem.org/Template:Stockholm/content-bundleJS?action=raw&ctype=text/javascript"></script></body></html> |