Overview
MIKROSKIN aims to be an easy to use rapid test for the detection of dysbiosis in the skin microbiota. To develop our rapid test, we divided the project into smaller parts, to make the development easier and faster, as we were able to work on several parts simultaneously. Our project is divided into three different parts.
The first part is the aptamer selection, where we use SELEX to select aptamers against the common skin bacteria C. acnes and one aptamer against li[pteichoic acid, present in the wall of all gram-positive skin bacteria. The second part of the project is the analysis of an already discovered aptamer against S. aureus. The third part is the development of the detection method, where we used the colorimetric properties of the polymer PCDA to induce a colour change upon aptamer-target binding.
Our goal for our project was to develop a prototype of our rapid-test, which we could use in presenting our product to potential stakeholders.
Aptamer Selection
The aptamer selection was done by using cell-SELEX for C. acnes in a bead based-SELEX for protein A. Both protocols needed optimization to make them work properly. We obtained results for both protein A and C. acnes in the first SELEX round, showing that some inital library aptamers bind to our targets. However, due to time shortage, we did not manage to show an increase in affinity over multiple SELEX rounds, therefore we could not increase the affinity of our library.
Binding affinity should be shown by using FACS analysis. The fluorescent tag of the aptamer enables to show target binding by measuring fluorescence intensity, as done by Van Simaeys et al (1).
Aptamer Testing
To develop a functional and highly sensitive rapid test, it is important to have sensitive aptamers. We analysed the binding affinity with three different approaches:
- Affinity chromatography
- Bead based purification
- Fluorescent detection.
Affinity Chromatography
Chromatography is a widely used technique to test aptamer target binding (2). However, in our experiment no binding of the protein A aptamer to the HiTrap Mabselect protein A affinity chromatography column was detectable.

Figure 1: Chromatography results. Chromatography was performed using the HiTrap Mabselect SuRe protein A affinity chromatography column. Top part shows the chromatogram. y-axis displays UV-absorbance and conductivity and x-axis displays volume in mL. Lower part shows 2% agarose gel of chromatography fractions run in TBE electrophoresis. Fractions 1-4 are from sample application. Fractions 15-24 are samples from elution.
Bead-based Purification
An alternative approach to the chromatography is magnetic-bead based affinity analysis (3). The aptamer affinity was tested using magnetic beads conjugated with protein A. Again, no binding of the protein A aptamer was detected.

Figure 2: Results of bead-based purification. Protein A products were run in 2% agarose gel shared with samples from a PCR troubleshooting. Wells 1-5 have PCR products with different annealing temperatures. Wells 6 and 7 show the product after elution of beads, before and after desalting.
Additionally, we decided to test the affinity of the aptamers additionally by using an affinity assay based on fluorescence detection. The affinity of the aptamers against the immobilized target were measured by detecting emitted fluorescence. This approach is highly sensitive in detecting target binding, and according to the calibration curve, low aptamer concentration in the pico molar range could potentially be detected.
Therefore, the protein A aptamer was tagged with a fluorophore on the 5' end.
The affinity of purchased protein A aptamer was tested as described. Unfortunately, the aptamer affinity to bind protein A was lower than 1%, which led us to conclude that the protein A aptamer affinity is remarkably low.
Detection Method
Our rapid test is based on a colorimetric change induced by the aptamer binding its target. Therefore, the aptamer is coupled to PCDA vesicles. Target binding induces mechanical stress to the PCDA vesicles, which induces a conformational change, thereby shifting the PCDA colour from blue to red (1-2).
We successfully synthesized PCDA vesicles and showed their colour changing ability due to external stress, in the form of heat and UV-light, as seen in Figure 3.
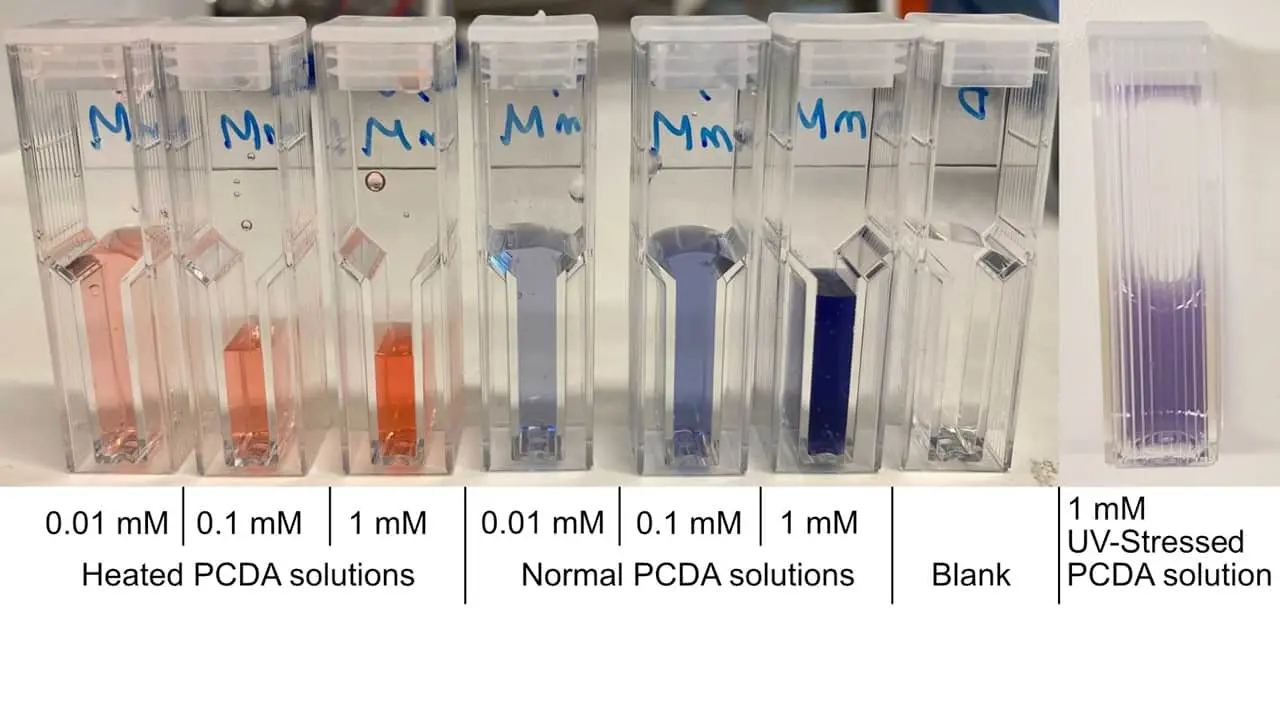
Figure 3: PCDA solutions subjected to different stress conditions (heat, UV radiation, normal), and subsequently diluted for spectrophotometric measurements
These colorimetric properties of PCDA vesicles are utilized in several developed biosensors. It could be shown by Wu et al. that PCDA vesicles conjugated to aptemers change colour upon target binding (3-5).
We conjugated the PCDA vesicles with the protein A aptamer. However, no colour change was detectable. This is expected, because as previously mentioned, the binding affinity of the aptamer was very low.
Functional Testing
The final step in the project is the functional testing of the PCDA-Aptamer conjugates on facial swabs. This step is necessary to show that the detection method is working under application conditions. However, this step was not conducted due to time shortage.
Conclusion
In our project we aimed to develop an aptamer-based rapid test to detect skin bacteria in a semi-quantitative manner. We were able to develop an experimental procedure for the development of the rapid test. The developed protocols need further optimization to succeed in producing a functional test, however, we succeeded in obtaining promising results for some parts of the process.
After the successful optimization of the experimental procedures, we will develop a customizable rapid test with a wide range of aptamers. You can read more about our implementation strategy here
References
- Van Simaeys D, Lopez-Colon D, Sefah K, Sutphen R, Jimenez E, Tan W. Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PloS one. 2010 Nov 1 ; 5 (11) : e13770
- Perret G, Boschetti E. Aptamer affinity ligands in protein chromatography. Biochimie. 2018 Feb 1 ; 145 : 98 - 112
- Zhu G, Walter JG. Aptamer-modified magnetic beads in affinity separation of proteins. InAffinity Chromatography 2015. Humana Press, New York, NY.
- Jung YK, Kim TW, Park HG, Soh HT. Specific colorimetric detection of proteins using bidentate aptamer conjugated polydiacetylene liposomes. Advanced Functional Materials. 2010 Sep 23 ; 20 (18) : 3092-7
- Lee J, Jun H, Kim J. Polydiacetylene–liposome microarrays for selective and sensitive mercury (II) detection. Advanced Materials. 2009 Sep 25 ; 21 (36) : 3674-7
- Wen JT, Bohorquez K, Tsutsui H. Polydiacetylene-coated polyvinylidene fluoride strip aptasensor for colorimetric detection of zinc (II). Sensors and Actuators B: Chemical. 2016 Sep 1 ; 232 : 313-7
- Tobias A, Rooke W, Hanks TW. Incorporation of gold nanoparticles into the bilayer of polydiacetylene unilamellar vesicles. Colloid and Polymer Science. 2019 Jan 17 ; 297 (1) : 85-93
- Wu W, Zhang J, Zheng M, Zhong Y, Yang J, Zhao Y, Wu W, Ye W, Wen J, Wang Q, Lu J. An aptamer-based biosensor for colorimetric detection of Escherichia coli O157: H7. PloS one. 2012 Nov 7 ; 7 (11) : e48999
