
While conducting experiments using LbCas12a-RVRR, we found that it could not do multiplexing in one pot reaction. This limitation means that only one target gene can be identified in a single reaction, and we cannot find out different kinds of cancer mutations in the sample.
In addition, the inability to do multiplexing in one pot reaction, restricts us from using an internal control, which is important in ensuring the test is valid.
Hence, we decided to use a Cy5 conjugated probe to discriminate against the V600E mutations. From the data, we discovered that the probe was able to distinguish between mutations readily. This is supported by the figure below, where strong fluorescence was emitted in the presence of E600 mutant, and weaker fluorescence was detected in presence of V600 (wildtype).

As shown, the probe-based method is able to be done in a single LAMP reaction without the need to change to another temperature or another step for liquid transfer of LAMP product to the CRISPR reaction. This would simplify the protocols for testing and eliminate false results due to human error.
Why COVID-19
While cancer is a serious disease, the COVID-19 pandemic currently poses a more significant threat due to its high transmissibility [1]. The virus can spread quickly between people and may result in a rapid increase in the number of cases or deaths. This increases the demand for regular testing on a larger scale. Rapid detection also allows for quicker preventative measures, allowing the government to put measures in place to curb the spread of COVID-19.
About COVID-19
COVID-19 is a contagious respiratory disease caused by the SARS-CoV-2 virus which primarily spreads through airborne transmission [1]. As of 21 October 2021, there have been at least 242 million cases and 4.94 million deaths around the world [2].
What testing methods are currently available?
To reduce the severity of COVID-19 outbreak, several methods were developed for massive testing. Firstly, the Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) test is the main diagnostic test for the virus[3]. The Antigen Rapid Test (ART) is an alternative to RT-qPCR and is an over-the-counter self-test kit [4]. Lastly, the Antibody Test is a test used to detect the presence of antibodies against virus if one has been infected by the virus before, or have currently been infected [5].
What are their advantages and limitations?

What can address all these limitations?
Reverse Transcription-Loop Mediated Isothermal Amplification (RT-LAMP) based assays can be utilised. They are able to address these limitations of the RT-qPCR and ART.

Nevertheless, there exist disadvantages of RT-LAMP such as the formation of primer dimers due to the larger number of long primers used in the reaction and false positives. While the amplification of the DNA has been successful, there is no indication on whether the target sequence is present.
How do we tackle the disadvantages of RT-LAMP?
After comparing CRISPR-based and probe-based experiments, we have come up with an assay using a probe targeting the S gene of SARS-CoV2 genome due to its ease of use. In our experiments, we show that with the incorporation of the probe, a sensitivity of ~20 copies per reaction can be achieved with up to 95% accuracy, similar to that of the RT-qPCR. Therefore, this method blends the advantages of RT-qPCR and ART, while addressing all the limitations listed above.

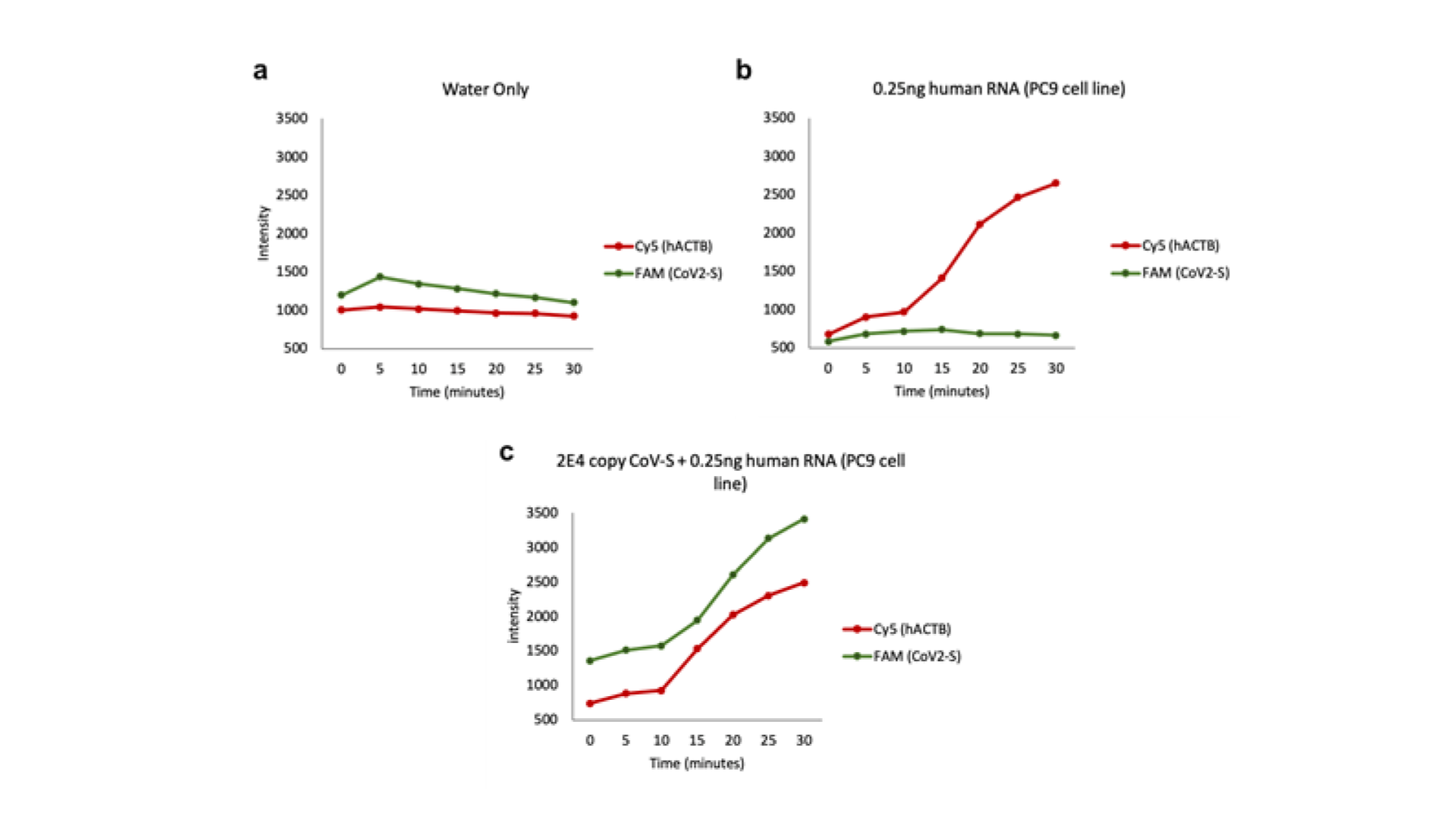
In addition to the probe being able to be carried out in a one-pot reaction, it shows properties of being able to be used for multiplexing, allowing us to add an internal control to the assay.

As seen from the figures above, when both probes were added into the sample containing only saliva, the Cy5 signal was apparent. When both probes were added into the RNA solution, only the FAM signal was apparent. When both probes were being added into the RNA with saliva, we were able to observe both Cy5 and FAM signals. Given the data obtained, we can now embark on the designing of our prototype device.


- “Coronavirus disease (covid-19): How is it transmitted?,” World Health Organization, 13-Dec-2020. [Online]. Available: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted. [Accessed: 21-Oct-2021].
- “Coronavirus cases:” Worldometer. [Online]. Available: https://www.worldometers.info/coronavirus/. [Accessed: 21-Oct-2021].
- “Why qPCR is the gold standard for covid-19 testing,” Ask A Scientist, 01-Jul-2021. [Online]. Available: https://www.thermofisher.com/blog/ask-a-scientist/why-qpcr-is-the-gold-standard-for-covid-19-testing/[Accessed: 21-Oct-2021].
- “Educational Resources on Antigen Rapid Test,” MOM. [Online]. Available: https://www.mom.gov.sg/covid-19/educational-resources-on-art. [Accessed: 21-Oct-2021].
- “Antibody (serology) testing for covid-19,” Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers, 19-May-2021. [Online]. Available: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/antibody-serology-testing-covid-19-information-patients-and-consumers. [Accessed: 21-Oct-2021].
- B. May, “Pros and cons of the common types of COVID-19 tests,” BioSpace, 15-Oct-2020. [Online]. Available: https://www.biospace.com/article/pros-and-cons-of-the-common-types-of-covid-19-tests. [Accessed: 21-Oct-2021].
