
Implementation
In recent years we have seen how daring ideas have become reality, fueled by the means of synthetic biology. However, from the success in engineering a microorganism, till its application within a real context there is a long way to drive. Within the bio-manufacturing industry, process developments and scale-up are the toughest challenges to face. In this page you will find information about how we can go from the test-tube to the industry.
Implementating Photobiocatalytic Technology
During our project we have carefully analyzed what are the main challenges of the current phototrophic microorganisms based industry. After that we decided to develop a novel approach for photosynthetic biomanufacturing: The utilization of microorganisms as living catalysts, instead of mere “resources” to cultivate.
Our aim is to study how our engineered microorganism could be used as the heart of an industrial process for the sustainable manufacturing of sustainable biofuels and other chemicals.
How could our technology be implemented within Industry?
The Challenges within Microalgae Industry
Microalgae industry is a wide definition which refers to any biotechnological installation whose purpose is the manufacturing of valuable products using or cultivating phototrophic microorganisms. Despite the big attractives of phototrophs to directly harvest light and carbon dioxide and convert them into valuable products, there are several pending challenges which are slowing down their large-scale implementation.
We could think that the microalgae industry will bloom especially in those regions where sunlight and water are highly available, however a big part of the microalgae industry is settled in cold regions, and sometimes they even grow the microalgae indoors with artificial lights. Most of these microalgae-based companies focus their strategy in the production of high added value compounds like pharmaceuticals or nutritional supplements, since the high selling costs of their products can compensate for the expensive costs of production. However, if we are planning to unravel the real potential of phototrophic microorganisms, using them for the large-scale production of industrial chemicals “out of the air” , we can not ignore the current problems that are impeding microalgae industry develop, specially in regions where their geographical features are ideal for their installation.
The Challenges
Large-Scale Cultivation
Unlike other microorganisms, the large-scale biotechnological application of microalgae/cyanobacteria is recent. This means that industrial cultivation technologies are not as developed as the fermenters used for heterotrophic cultivation. Consequently, the productivity as well as the costs of installation, use and control of the cultivation systems is still an area in need of major optimization.
Phototroph industrial cultivation for biomanufacturing requires a smart design. These challenges are the implementation of efficient light harvesting design, adequate systems for providing CO2 to the cells as well as strategies to prevent culture contaminations and reducing the spontaneous mutation of engineered strains.
Downstream Processing
Current industry relies on the cultivation of microorganisms for its further harvesting and extraction of one or more products. This implies that cells should be separated from the liquid culture media, concentrated and processed for the extraction of desired compounds or the stabilization of the biomass for its direct usage.
Within the current industry, the operations required for cell harvesting and further stabilization or extraction of products can account for up to 85% of the total energy and resources consumption of the process.
This is the main reason why the microalgae based industry has not stepped into the mass production of chemical products, since most of the time, the resources required to generate the product are simply more than the intrinsic value of the produced material.
Biomass centered Industry
Following the paradigm of traditional biotechnology, the microalgae industry is mostly based in the cultivation of microalgae for the exploitation of their biomass and the compounds produced by the cells. Either if a metabolite is kept inside the cells or secreted to the liquid media, the vast majority of processes are based on the same principles.
These principles can be summed up as:
- Cultivate as fast as possible to produce more.
- Separate the cells from the liquid to obtain two fractions: Biomass and supernatant.
- Process the supernatant and biomass to extract the products of interest, where sometimes, further refining steps of these materials is required to generate the final product.
This conventional procedure has one big issue, still considering the cells as a resource to exploit, the higher biomass yield, the higher product you will recover. However, if we take into consideration the high requirements of biomass cultivation, harvesting and processing, it starts to be understandable why it is so difficult to achieve a viable process.
In addition, if your production target is a specific compound, the produced biomass is actually a process sub-product which is actually capturing most of the carbon and materials used as feedstocks. This scenario is typically found within high added value products where despite the low carbon conversion efficiency the mere benefits derived from product sellings make them viable.
But… Are these processes actually sustainable?
Almost any industrial process utilizing phototrophic microorganisms nowadays follows one key rule: engineering the production process to meet the requirements of the employed microorganism. However, in the age of synthetic biology… Could it not be possible to engineer a microorganism in order to adapt the requirements of industry?
After learning about the reasons that are slowing down the microalgae industry to provide the world with a sustainable way to manufacture the products we need, we decided to propose an alternative technology that could overcome its limitations for bulk-chemicals production.
Implementing a different technology: Photobiocatalytic Bioprocesses
To develop a technology capable of solving the main challenges of the microalgae industry we have taken a holistic perspective which considers simultaneously all the factors involved within the industrial production process. Embracing the knowledge of many different knowledge fields, our proposal relies on three main interrelated aspects
Engineering Photosynthetic Biocatalysts
Instead of growing biomass that should be harvested and processed with high energetic costs, we bet on a different strategy: using microorganisms as living catalysts. We aim to engineer a system where the desired products are directly synthesized by our engineered cyanobacteria and actively secreted outside the cells, significantly easing their recovery. To know more visit our Metabollic Engineering Page. Using this approach, the biomass should not be processed for the extraction of certain compounds that usually require further upgrading (like the case lipids in algae-based biofuels). However, even in this scenario, cells will continue growing and biomass separation will be required. Unless… that the product could be recovered by other means.
Process Engineering
Within our project, our goal is the production of an advanced biofuel: n-butanol. As well as many other important chemicals, n-butanol is volatile, which means that it can be recovered directly from the culture media as a vapour. For each product a different purification strategy should be designed, however, even with cells that are constantly dividing, if the targeted product is directly secreted into the media, the overall simplicity of downstream processing is enhanced. However, many other valuable industrial products like ethylene, terpenes or light alcohols can be easily recovered in the gas phase. Our particular case, n-butanol, the volatility is rather low when compared with the former ones, then we have explored different alternatives that could be also suitable for its adaptation to many other industrially relevant chemicals. To learn more about the design of our n-butanol production process, visit our Proof of Concept page.
Designing Photobiocatalytic Materials
Phototrophic organisms tend to grow rather diluted, as a naturally evolved trait to avoid excessive light absorption and shading effects. This raises the complexity of cell harvesting and increases the required size of cultivation systems. To solve these and other problems found in industry we have decided to develop a truly photobiocatalyst, a material capable of using sunlight to convert carbon dioxide into a biofuel like n-butanol. The utilization of a macroscopic and easy to handle material with engineered cells trapped inside pose multiple advantages for its industrial application. The most evident is the easier separation from the culture media and reducing drastically the high costs associated with biomass harvesting. Other benefits are the possibility of extended utilization times, protection of the cells from the harsh industrial environment and even the increase in productivity since cell division is limited. To know more about the development of these photobiocatalytic materials, visit our Encapsulation page.
Taking into consideration all of the above, we have evaluated different process schemes that will allow the creation of a viable industrial process.
Technology Scale-UP: Designing a Bioprocess
When working in the lab we are all concerned about guaranteeing the best conditions possible for our experiments. We always try to generate the perfect environment for our system to show its best performance. During the research process we overlook many small details, like the way we cultivate microorganisms or what kind of materials we use for keeping them alive. While for the lab-scale these little things have no really big importance, when thinking about scaling up they start to matter. It is easy for us to grow some milliliters of a culture within a flask, however how will you culture several thousands liters of bacteria? Building a giant flask is clearly not a choice and phenomena like heat or mass transfer start gaining much more importance than in the small scale.
Engineering microorganisms for bio-manufacturing is an amazing idea, but the validation of microorganism’s abilities to synthesize a product is just the beginning. The way the potential of the engineered microorganisms can be harnessed is via the development of a bioprocess.
The consolidation of a Bioprocess involves the consideration of multiple aspects from an holistic point of view. A Bioprocess can be seen as an ordered scheme of multiple sequential operations, that allows the conversion of certain inputs (raw materials, energy etc…) into a desired output (bioproduct), where a biological system is usually involved in the hearth of materials conversion step.
Within Industry, viability is the key word. If all the costs derived from the operations required to keep working the process overcome the benefits derived from the product obtained, it will never be developed. The same can happen If the required technologies have not yet been adapted to the industrial scale. Then a bioprocess should not only be economically viable, but also technologically.
Every bioprocess can be divided into three main stages: Pretreatments, Conversion and Downstream. The engineered microorganisms are typically found in the reaction stage; the core of the process.

During the pretreatment stage, feedstocks and other required materials are conditioned to the parameters required for the conversion step. In the case of our design, this corresponds to the pressurization of the air for aeration and culture mixing. The preparation of growth media and its conditioning till reaching the required temperature are also part of this stage.
In the conversion step, the engineered microorganism performs as a biocatalyst, converting the materials supplied to the reaction system into the desired products. In this step, the conditions should be carefully controlled to achieve the optimal productivity.
In the downstream stage, the product is recovered after the conversion, being separated from the microorganism and isolated from the rest of the compounds previously present or generated during the biological process.
Upstream & Reaction: Photobioreactor Design
Regarding the initial stages of a bioprocess, the microalgae industry has one interesting feature: The required substrates are minimal. While in other bioprocesses multiple feedstocks need to be processed and conditioned, phototrophic microorganisms just require some salts dissolved in water, light and air. This way, the upstream stage is almost directly coupled within the conversion process. All of these operations are performed within a Photobioreactor system.
What is a photobioreactor
Briefly, a photobioreactor (PBR) is a system where biological systems capable of light utilization are grown microalgae or cyanobacteria in most of the cases. A photobioreactor system is usually divided into the culture chamber (where the cells are cultured) and the auxiliary equipment required for its proper functioning (pumps, air compressors, heating elements etc…).
Depending on the requirements of the strain to culture, and more importantly, the overall process requirements different photobioreactor systems can be employed. Two main PBR categories can be defined.
Open Systems

These are the simplest and cheapest PBR design, however the control of culture conditions is limited and special measures have to be taken in order to avoid contamination of the culture.
Raceways and open ponds are phototrophic microorganisms cultivation systems that usually have large volumetric capacity and are placed outdoors in areas where the ambient conditions are ideal for microorganisms growth.
They are mostly used for water treatment or the cultivation of strains that pose special features that allow them to thrive in environments where the chances of biological contaminations are greatly reduced. Examples of these systems are the open ponds utilized for the cultivation of Dunaliella salina.
Their major disadvantages are the lack of control of the cultures, big risk of contaminations, high water evaporation and the reduced performance of the microorganisms due to these factors.
Closed Systems

Closed photobioreactors are prefered for the cultivation of engineered microorganisms. They allow a tighter control of the culture conditions maximizing the performance of the cultured microorganisms for their required task. In these closed designs gas is supplied to the cultures as well as additional mixing or heating elements could be included.
The most common types of closed PBRs are, in increasing complexity: the flexible bag PBRs, the flat panels PBRs, the vertical columnar PBRs and the horizontal tubular PBRs. In general, the greater the system's capacity to control culture parameters, the higher the cost of installation and operation.
The horizontal cultivation systems aim to maximize the light availability for the cells. However, this makes them more complex. This way, horizontal tubular PBRs require pumps for keeping the culture continuously moving through horizontal transparent tubes. In the middle of the circuit, a wider vertical tube is found where pressurized air is bubbled, allowing for gas exchange. Sometimes even temperature control elements are included. This way, pumps, compressors and heating elements are required and permanently active.
Other PBR designs like the vertical photobioreactors try to find a compromise between the advantages of closed systems and simplicity. Among them Flat Panels aim to be the ideal choice for large-scale industrial utilization. They consist of vertical or inclined hollow panels with a depth lower than 20 cm in order to avoid shading effects. Phototrophic microorganisms are cultivated within the panel and pressurized air is pumped from the bottom, providing simultaneously gas exchange and efficient culture mixing.
Among all the currently used industrial reactors, flat panel designs are the most promising candidates for the large-scale production of chemicals. Because of its design, this type of PBRs has a high surface, maximizing its exposure to light, even when placed vertically. In addition their design is easy to manufacture and robust, and the required auxiliary equipment is limited.
However, any of the former PBR designs still have their own drawbacks… and industry is currently moving forward to provide new solutions.
Optimizing photobioreactor designs
The most differential feature of a photobioreactor is the requirement of light exposure. The requirement for efficiently harvesting light makes them difficult to engineer in the same way as traditional fermenters used in the rest of the biotechnological industry. However, there are some important aspects that could be considered to greatly improve photobioreactors attractive for industrial applications.
The Surface-volume challenge
Conventional bioreactors are usually a tank provided with agitation, heating and aeration equipments, everything required by the organisms grown inside is provided with the culture media or aeration. However… Phototrophs also require light, and this makes the engineering of PBRs totally different.
It is easy to gather several thousands of liters within an industrial fermenter, however this is not applicable to phototrophs. They require light, then cells in the outer parts of the system will shade the ones lying behind. Even if adequate culture mixing is provided, depths higher than 0,2m are enough to start observing the shading effect. Shading implies that not all the cells present within the culture are actually optimally performing their functions, since they do not receive as much light as they would require. To avoid this shading effects, all PBR designs tend to increase it’s surface to volume ratio. This is the reason why we don’t have transparent tanks for growing microalgae.
Photobioreactors are bidimensional (2D) cultivation systems, while the rest of the industry is used to the conventional tridimensional fermenters, where the most important parameter is the volume of the system. This two-dimensional nature poses a serious problem for the industrial deployment: a lot of surface area is required. For cultivating the same volume that in a traditional fermenter it could fit in less than some square meters, you will require a vast extension of land. And this also implies that wires, pipes and other necessary auxiliary equipment should be distributed along all the installations. This situation greatly increases the investment cost of any phototrophic-based industrial facility.
After researching deeply we have found several proposals that aim to overcome these limitations. Could it be possible to grow phototrophic microorganisms in 3D systems instead of the conventional 2D-PBRs? Definitely Yes! Utilization of Light Distribution systems. (h5) In the beginning of our research we thought about the possibility of utilizing optic fibers for the efficient light distribution within cultures. In fact, we found some authors that have proposed alternative systems for efficient light distribution.
The utilization of optic fibers or other light distribution elements within a PBR design is a powerful idea that could change the way we currently conceive PBRs. Besides allowing for a more compact culturing system, it will also open the doors for other promising improvements.
Of course external light harvesting and redirection systems should be designed, in order to transfer this light to the optic fibers. But these systems could be even used for “concentrating” or “diluting” the amount of light transferred to the culture, allowing for a more efficient utilization of the available energy. In addition, this light distribution element can be also fed with artificial light coming from LEDs or other light sources. Which could greatly enhance not only the culture performance, but also open the possibility to a wide range of options for photo-regulation of the culture.
However, despite the hype that these systems may pose, they have only been studied at small scales. In addition, challenges like avoiding the fouling of light distribution elements or the intrinsic complexity of their construction makes them just an attractive but future possibility for the current microalgae industry.
Intermittency of daylight
Another crucial aspect of phototroph cultivation is the availability of light. In contrast with other organisms, they rely on light availability to perform their functions. However, these limitations could be overcomed by new developments.
Storing light for later
Photovoltaic technologies could allow us to efficiently harvest light during the days and store it as electricity for later use in providing the cultures with light during the dark or in cloudy days. The new developments on robust electric storage technologies, like liquid metal batteries or supercapacitors could provide a reliable energy source for keeping the culture permanently active. In addition, the combination of these artificial illumination systems with other strategies as the formerly mentioned could enhance the efficiency of the overall process. With the utilization of these technologies, phototrophic based production could be constant and more reliable, becoming comparable with other industrial processes.
Looking closer to the Investment Costs
Despite the technical viability of the proposed systems, the current challenge is making them competitive for the industry. They are new ideas and prototypes that have not been fully tested at the large scale level. Because of this, the investment costs of these technologies is very high, and despite its promising potential, industry will continue preferring the already existing cheaper options, until these new approaches starts to reduce their price.
In any case, for a production process based on phototrophy, cultivation is not the most critical part but the posterior downstream operations. Then, since our purpose is to develop a technology that could be rapidly implemented within the current industry, we have decided to select a conventional flat panel photobioreactor design.
The challenge of Downstream Processing
As formerly mentioned, depending on the type of microorganisms, product and cultivation system, downstream processing can account for 40-85% of the total production costs. Our aim is to simplify the downstream process, reducing the energy consumption and required equipment and materials typically used for product recovery.
Then, the first step to design an adequate recovery and purification process is to know about the product.
Our Product: n-butanol
n-Butanol is a medium chain alcohol widely used in industry for many different applications. It can be used not only as a biofuel but also as an intermediate chemical in the manufacturing of pharmaceuticals, artificial leather, textiles, safety glass, rubbers, cement or perfumes among many others.

In order to recover butanol, we should consider its chemical properties. It is a moderately volatile compound, with low vapor pressure at ambient conditions that rapidly increases with temperature. It is partially miscible with water, where at concentrations higher than 72 g/L two phases are formed. Likewise it forms an azeotropic mixture with water at 55.5%wt. It is also partially hydrophobic due to it’s longer hydrocarbon chain than other alcohols. That is another interesting property that expands the possibilities for its recovery via its interaction with hydrophobic materials.
Available technologies
In order to develop an efficient downstream process, a different combination of operations must be defined. Each one of these operations correspond with a physicochemical process that allows to separate n-butanol from other materials. Fortunately the chemical industry has a vast experience in the separation of complex mixtures. The wide range of technologies once developed for oil refining can today be applied for the development of more sustainable production processes.
Then… What are the main technologies that could be used for n-butanol purification?
Product Recovery & Purification Technologies
Distilation
Thermal separation based on the relative volatility of the mixture components.
It is a highly optimized operation which stands for the industrial standard for volatile compounds purification. However, it is not suitable for highly diluted mixtures due to the high energy consumption required for vaporization of the mixtures. In this operation the liquid mixture which contains the product is evaporated and condensed succesively, separating its components according to is volatility. During the operation, reboiler works at higher temps than condenser which does not allow for efficient heat recovery during the operation. However, the condenser of a distillation tower could be used as a heat source for other requirements in the process.

Gas Stripping
Volatilization of the desired compounds relying on liquid-vapor equilibrium.
Briefly, gas stripping consists in the removal of a volatile compound by means of continuous removal of the vaporized product in accordance with its vapour pressure. The challenge of moderate volatility of n-butanol requires high temperatures for an efficient recovery.However the possibility of combining stripping with culture aeration makes it a promising approach. The concentration would be really low and could require intensive energy use for condensation.

Extraction
Separation based on product affinity for a second liquid immiscible phase.
Extraction consists in the selective distribution of a desired products between two different inmiscible substances. Liquid-liquid extraction is another well established industrial operation for the recovery of butanol. It does not require intensive energy consumption, since the contact between the liquid feed and the solvent is enough to drive the extraction process. However, when highly diluted butanol streams needs to be extracted, enormous equipment and high consumption of solvents (that should be regenerated) are required. Because of this extraction could not be a really efficient solution so far.

Adsorption
Separation based on selective surface interactions of an adsorbent material with the product.
Adsorption consists in the selective distribution of a compound between a fluid phase and the surface of a solid material. These feature the great advantage of being more independent on product concentration in the feed. Adsorption processes are often to purify streams from products found at very high dilutions. This way, adsorption allows to generate highly concentrated products from very low feeds without severely affecting energy consumption. However the main challenges is the identification of good adsorbents, which must be highly selectivity towards butanol, reducing the issue of co-adsorption of similar compounds. Nevertheless, on the contrary of conventional fermentative n-butanol processes, when cyanobacterial cultures are used, the ammount of potential contaminants is greatly reduced.

Per-Evaporation
Membrane Separation based on selective diffusion and adsorption.
In pervaporation, the medium with a low product concentration is separated by partial vaporization through a selective membrane, which can be integrated with the reaction system or operated independently. The butanol culture medium comes into contact with the hydrophobic membrane and diffuses from one side into the membrane. The compounds are extracted from the opposite side of the membrane by application of vacuum and recovered at a high concentration by condensation.
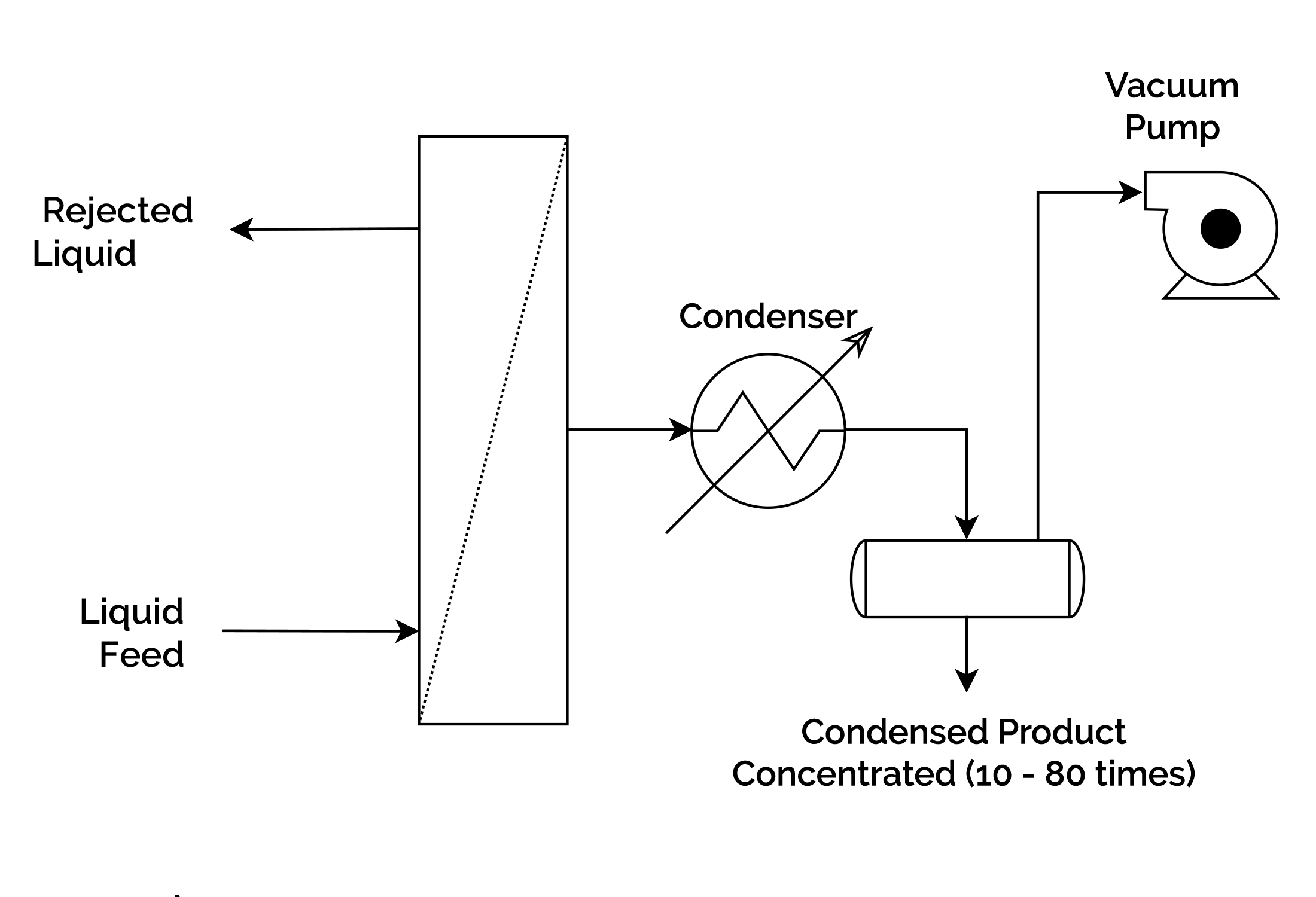
The main limitations of this process are the low flux through the membrane, the high membrane surface areas required and the fragility of the membranes. Phenomena such as bio-fouling or swelling are also relevant. Because of this, the operation is usually carried out in a separate system from the bioreactor, so that the retention of the cells in the reaction system or their separation by microfiltration/centrifugation is required prior to their entry into the per-evaporation unit.
The ratio of selective diffusion of the compound in the membrane and diffusion through the membrane determine the selectivity and permeability of the membrane. Membrane thickness, vacuum, feed composition or temperature are some of the parameters that define this operation. Silicone or silicalite-silicone membranes, polymeric, composite or liquid supported membranes are the most common options.
Designing an efficient downstream process
Considering the available technologies, we have designed different downstream purification schemes and evaluated them in terms of their energy consumption, materials requirements, complexity and capital investment, as well as the achievable product purity under realistic operational conditions.
In addition, for designing all of the processes, we have considered the advantages derived from the usage of cells as photobiocatalysts instead of biomass. To do so 2 different scenarios of utilization has been taken into account
First scenario contemplates the normal cultivation of engineered microorganisms, allowing for cell division. In these cases, product recovery should be preferably achieved without the need of energy-intensive biomass separations.
The second scenario considers the utilization of photobiocatalytic materials that allow for an eased separation. Although THis approach will require a previous cultivation and biomass separation step, the extended lifespan of the biocatalyst and its improved properties could still improve the overall process viability.
Designing Alternative Downstream Process
Centrifugation > Liquid Adsorption > Desorption > Condensation
In this process cells are separated by centrifugation, while the supernatant is fed to an adsorption unit composed of two parallel fixed beds. A similar alternative is proposed for photobiocatalytic materials in process 1B.

After the saturation of the adsorption bed, the supernatant is redirected to the second bed and the former one enters into the desorption process, where hot air at 130 ºC is used to desorb the n-butanol. First, the hot air will heat the bed, drying the excess of not adsorbed water which will remain wetting the column. In addition, this hot stream can be heat exchanged with the growth media for its conditioning before feeding the reactor. The composition of the desorption stream is analyzed, and once the bed has been fully dried, it is submitted to a condenser, where the n-butanol is recovered with a purity between 94-97%. During this desorption stage, the gas exiting the condenser would be partially redirected to the column fed.
Within this process, biomass would be a sub product that could be furtherly valorized.
Sedimentation > Liquid Adsorption > Desorption > Condensation
In this process the photobiocatalytic material can be recovered by sedimentation or filtration. The clarified supernatant is fed to an adsorption unit composed of two parallel fixed beds. This process the usage of a photobiocatalytic material that can be easily retained in the photobioreactor or recovered via a simple filtration step.

After the saturation of the adsorption bed, the supernatant is redirected to the second bed and the former one enters into the desorption process, where hot air at 130 ºC is used to desorb the n-butanol. This hot stream can be heat exchanged with the growth media for its conditioning before feeding the reactor.
The composition of the desorption stream is analyzed, and once the bed has been fully dried from the excess of non-adsorbed water it is submitted to a condenser, where the n-butanol is recovered with a purity between 94-97%. During this desorption stage, the gas exiting the condenser would be partially redirected to the column fed.
LIkewise, the recovered photobiocatalysts could be fed back again to the cultivation system.
Per-Evaporation > Condensation.
In this process cells or photobiocatalyst does not require to be separated. However, a specialized photobioreactor design is required. A hollow tubular photobioreactor will be necessary. The culture will be held in an annular section in the outer part of the tube, while multiple membranes of different porosity will be intercalated between the culture and a per-evaporation membrane.
The application of vacuum within the inner part of the tube will drive the selective diffusion of butanol through the membrane and its evaporation within this internal space. Then, the butanol can be recovered via condensation of the stream exiting the per-evaporation system. Condensation can be performed at atmospheric pressure in order to enhance n-butanol recovery.

It is important to note, that Despite the existence of some per-evaporation reactors, where membranes are integrated within the cultivation system, the delicate nature of these materials will require additional care to avoid damaging the system. To avoid these problems, a variation of this process could imply an additional biomass separation step by centrifugation, allowing to perform the product recovery in a dedicated per-evaporation unit. However it will also increase the energy requirements
Gas Stripping > Gas Adsorption > Desorption > Condensation
This process relies on the integration of culture aeration with product recovery. The air stream employed for gas exchange will strip part of the dissolved n-butanol. Aided by the utilization of strains with high optimal temperatures (38ºC) the n-butanol will be transferred to the air stream.
This air stream is fed to an adsorption unit composed of two parallel fixed beds. After the saturation of the adsorption bed, the air stream is redirected to the second bed and the former one enters into the desorption process, where hot air at 130 ºC is used to directly desorb the n-butanol, since water is not retained in the fixed bed while gas-phase adsorption.
During desorption, this hot stream can be heat exchanged with the growth media for its heating before feeding the reactor.
A condenser will be used to recover the butanol from the desorption stream. The n-butanol is recovered with a purity between 94-97% depending on the desorption air humidity. During this desorption stage, the gas exiting the condenser would be partially redirected to the column fed, in order to increase the recovery yield.

This process is highly attractive because of its simplicity and integration within the reactor system, however due to the moderate volatility of n-butanol, high air flows will be required in order to achieve n-butanol recovery. Only with the utilization of highly tolerant engineered strains which won’t suffer from product inhibition, the high concentrations of n-butanol in the culture media will allow an efficient recovery.
Gas Stripping > Gas Adsorption > Desorption > Condensation
This process corresponds with a more traditional approach for the recovery of bioproducts. Initially cells are separated from the culture media by centrifugation. In this case a total clarification of the culture is not required.
Then a hydrophobic solvent like naphtha is mixed with the liquid within a countercurrent extractor, where via multiple liquid-liquid equilibrium stages, the solvent pulls most of the butanol out of the aqueous phase.
After that, organic and aqueous phases are separated into a decanter vessel, aqueous phase can be partially recycled for its cultivation, while the organic phase is submitted to distillation for simultaneous n-butanol and solvent separation. This way, solvent can be reused, while the obtained n-butanol will have a purity ranging from 90 to > 99%, however, higher the purity, bigger distillation equipment will be required and more energy should be employed.

The low concentration of n-butanol in the aqueous phase makes this process challenging. Although all the proposed operations are standard and can be highly optimized, the size and energy consumption of the required equipment could greatly exceed the energy the product contains by itself. This process will be used as a baseline case, which corresponds with the current industrial practices for the recovery of biobutanol from ABE broths (Acetone-Butanol-Ethanol fermentation with Clostridium species) or other similar products.
Downstream Process Evaluation Matrix
After gathering data relative to each operation and performing preliminary simulations of how the different processes could perform, we have created a decision matrix, where we have qualitatively ranked the features of each proposal considering 5 different levels for each feature.

The conformation of the process evaluation matrix allowed us to identify which scheme would be ideal for each one of the possible scenarios. As well as which advantages which downstream scheme is more suitable for an efficient n-butanol recovery.
We have selected the n-butanol adsorption processes as the most promising candidates whose performance have been explored in more detail within the Proof of Concept page.
The way towards industrial implementation
The conclusions
Implementation would be the last stage of the 4C_Fuels project. During this year we have performed a preliminary evaluation of the industrial requirements for a truly sustainable solar biomanufacturing technology.
We have identified downstream processes as a crucial element for industrial implementation. After designing and simulating multiple processes, we have selected butanol adsorption as a promising alternative. Then, we have performed a more detailed design of how a real installation will employ these technologies.
Based on the bioprocess design work we can provide an estimation of how the system will perform in the field, as well as state the critical features that an engineered phototrophic microorganism will require for a sustainable and viable industrial production. To know more about the estimation of the implementation, visit the Proof of Concept page.
Biological requirements
After performing the simulation of a bioprocess for n-butanol production, we have identified the biological engineering requirements for a viable biomanufacturing process.
The minimum viable product generation should be of 1.38 g*h-1*m2. A value which is currently under the performance of some already existing engineered strains! It is also important to note that product tolerance will define the final size required for an installation, being a critical factor in the investment cost of the technology.
However, the satisfactory implementation of an engineered strain will also require some additional features. An engineered cyanobacteria that can be used in the core of a biomanufacturing process should also be adapted to the harsh industrial environment. The strain must be easy to cultivate, feature a reduced contamination risk, and withstand a wide range of cultivation temperatures without severely compromising its productivity.
System Performance
Considering all of the above, a first estimation of the technology provides us with the following numbers.
Within a production plant with 10 m3 culture capacity, 10 m2 of surface will be used for light capture. Every day up to 4 L of butanol could be recovered. This butanol will provide 7 times the energy required for its production.
Of course, there is still a long road to pave, especially in terms of engineering better producing strains, capable of increasing the amount of product recovered per volume of photobioreactors. However the overall process is viable and while better strains and new smart engineering solutions for industrial cultivation of phototrophs appear, the lower the investment requirements for its large-scale industrial implementation.
Towards an integrated biomanufacturing industry
After evaluating the energy requirements of the process we can conclude that it could be viable. However we believe that this type of biomanufacturing processes should not be considered as an “isolated” entity, but combined with other technologies to achieve truly sustainable production. The utilization of wind or photovoltaic energy will be an essential requirement to sustainably power the photobioreactor systems and product recovery steps.
We believe in a technology capable of channelizing energy from the environment towards the biological conversion of carbon dioxide. Currently, photosynthetic microorganisms are our best tool for storing light energy into organic products. However, speaking about energy production, in order to cover the energetic requirements of the process, we must rely on the available sustainable energy harvesting technologies, whose efficiencies for solely energy production greatly exceed photosynthesis.
To sum up, any technology has its own advantages and while sustainable energy technologies are great at producing energy, they are not capable of dense energy storage for long term or providing us with materials. On the other hand, photosynthetic biomanufacturing allows us to harness light energy for carbon capture, and despite its relative inefficiency for energy conversion, it is great for dense energy storage and the production of materials “out of the air”. Then a truly sustainable biomanufacturing technology must take the best of both worlds.
Seader, J. D., Henley, E. J., & Roper, D. Keith. (2011). Separation Process Principles with Applications using Process Simulators.
Kuan, D., Duff, S., Posarac, D., & Bi, X. (2015). Growth optimization of Synechococcus elongatus PCC7942 in lab flasks and a 2-D photobioreactor. The Canadian Journal of Chemical Engineering, 93(4), 640–647. https://doi.org/10.1002/CJCE.22154
Myers, J. A., Curtis, B. S., & Curtis, W. R. (2013). Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophysics 2013 6:1, 6(1), 1–16. https://doi.org/10.1186/2046-1682-6-4
Cousin Saint Remi, J., Baron, G., Denayer, J., 2012. Adsorptive separations for the recovery and purification of biobutanol. Adsorpt. 2012 185 18, 367–373. https://doi.org/10.1007/S10450-012-9415-1
Incropera, F.P., DeWitt, D.P., Bergman, T.L., Lavine, A.S., 2017. Incropera’s principles of heat and mass transfer. Wiley 1000.
García Rodríguez, Á., García Rodríguez, Á., 2017. Recuperación de biobutanol para la producción de combustibles mediante ciclos de adsorción-desorción.
Magdaong, J.B., Ubando, A.T., Culaba, A.B., Chang, J.S., Chen, W.H., 2019. Effect of aeration rate and light cycle on the growth characteristics of Chlorella sorokiniana in a photobioreactor. IOP Conf. Ser. Earth Environ. Sci. 268, 012112. https://doi.org/10.1088/1755-1315/268/1/012112
García Rodríguez, Á., García Rodríguez, Á., 2017. Recuperación de biobutanol para la producción de combustibles mediante ciclos de adsorción-desorción.
Consider that the packed column is originally at a solute concentration cA (in the interparticular gas phase) [WWW Document], n.d. URL https://web.fe.up.pt/~lepae/simsorb/SMT.html (accessed 10.14.21).
Kommareddy, A.R., Anderson, G.A., Gent, S.P., Bari, G.S., 2013. The Impact of Air Flow Rate on Photobioreactor Sparger/Diffuser Bubble Size(s) and Distribution. Am. Soc. Agric. Biol. Eng. Annu. Int. Meet. 2013, ASABE 2013 6, 1-. https://doi.org/10.13031/AIM.20131620764
Seader, J.D., Henley, E.J., Roper, D.K., 2011. Separation Process Principles with Applications using Process Simulators. 849.
Myers, J.A., Curtis, B.S., Curtis, W.R., 2013. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013 61 6, 1–16. https://doi.org/10.1186/2046-1682-6-4
Kuan, D., Duff, S., Posarac, D., Bi, X., 2015. Growth optimization of Synechococcus elongatus PCC7942 in lab flasks and a 2-D photobioreactor. Can. J. Chem. Eng. 93, 640–647. https://doi.org/10.1002/CJCE.22154
Szepessy, S., Thorwid, P., 2018. Low Energy Consumption of High-Speed Centrifuges. Chem. Eng. Technol. 41, 2375–2384. https://doi.org/10.1002/CEAT.201800292
S, X., Q, Z., X, W., C, Y., W, C., 2013. A novel photobioreactor structure using optical fibers as inner light source to fulfill flashing light effects of microalgae. Bioresour. Technol. 138, 141–147. https://doi.org/10.1016/J.BIORTECH.2013.03.156
Do Gasoline Based Cars Really Use More Electricity than Electric Vehicles Do? | Council on Foreign Relations [WWW Document], n.d. URL https://www.cfr.org/blog/do-gasoline-based-cars-really-use-more-electricity-electric-vehicles-do (accessed 10.9.21).
Vapor Pressure Calculation by Antoine Equation (1-Butanol) [WWW Document], n.d. URL http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe (accessed 10.9.21).
(44) So: Exactly How Much Electricity Does it Take To Produce A Gallon of Gasoline? | LinkedIn [WWW Document], n.d. URL https://www.linkedin.com/pulse/so-exactly-how-much-electricity-does-take-produce-gallon-paul-martin/ (accessed 10.9.21).
Cousin Saint Remi, J., Baron, G., Denayer, J., 2012. Adsorptive separations for the recovery and purification of biobutanol. Adsorpt. 2012 185 18, 367–373. https://doi.org/10.1007/S10450-012-9415-1
Martin-Calvo, A., Perre, S. Van der, Claessens, B., Calero, S., Denayer, J.F.M., 2018. Unravelling the influence of carbon dioxide on the adsorptive recovery of butanol from fermentation broth using ITQ-29 and ZIF-8. Phys. Chem. Chem. Phys. 20, 9957–9964. https://doi.org/10.1039/C8CP01034J
Cousin Saint Remi, J., Rémy, T., Van Hunskerken, V., van de Perre, S., Duerinck, T., Maes, M., De Vos, D., Gobechiya, E., Kirschhock, C.E.A., Baron, G. V., Denayer, J.F.M., 2011. Biobutanol Separation with the Metal–Organic Framework ZIF-8. ChemSusChem 4, 1074–1077. https://doi.org/10.1002/CSSC.201100261
Rafa, N., Ahmed, S.F., Badruddin, I.A., Mofijur, M., Kamangar, S., 2021. Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front. Energy Res. 9. https://doi.org/10.3389/FENRG.2021.749968/FULL
Beardall, J., Raven, J.A., 2013. Limits to Phototrophic Growth in Dense Culture: CO
Du, W., Jongbloets, J.A., Pineda Hernández, H., Bruggeman, F.J., Hellingwerf, K.J., Branco dos Santos, F., 2016. Photonfluxostat: A method for light-limited batch cultivation of cyanobacteria at different, yet constant, growth rates. Algal Res. 20, 118–125. https://doi.org/10.1016/J.ALGAL.2016.10.004
Ding, Y., Guo, X., Yu, G., 2020. Next-Generation Liquid Metal Batteries Based on the Chemistry of Fusible Alloys. ACS Cent. Sci. 6, 1355–1366. https://doi.org/10.1021/ACSCENTSCI.0C00749
Liquid Metal Batteries - Helmholtz-Zentrum Dresden-Rossendorf, HZDR [WWW Document], n.d. URL https://www.hzdr.de/db/Cms?pOid=40421&pNid=226 (accessed 12.1.21).
Zhang, S., Liu, Y., Fan, Q., Zhang, C., Zhou, T., Kalantar-Zadeh, K., Guo, Z., 2021. Liquid metal batteries for future energy storage. Energy Environ. Sci. 14, 4177–4202. https://doi.org/10.1039/D1EE00531F
Hincapie, E., Stuart, B.J., 2015. Design, construction, and validation of an internally lit air-lift photobioreactor for growing algae. Front. Energy Res. 3, 65. https://doi.org/10.3389/FENRG.2014.00065/BIBTEX
Ponte, D.A.M.P. da, Werneck, M.M., Aranda, D.A., Ponte, D.A.M.P. da, Werneck, M.M., Aranda, D.A., 2016. Advances for Opaque PBR Internally Illuminated for Fiber Optic for Microalgae Production. Nat. Sci. 8, 341–358. https://doi.org/10.4236/NS.2016.88040
Chen, C.Y., Saratale, G.D., Lee, C.M., Chen, P.C., Chang, J.S., 2008. Phototrophic hydrogen production in photobioreactors coupled with solar-energy-excited optical fibers. Int. J. Hydrogen Energy 33, 6886–6895. https://doi.org/10.1016/J.IJHYDENE.2008.09.014
Xue, S., Zhang, Q., Wu, X., Yan, C., Cong, W., 2013. A novel photobioreactor structure using optical fibers as inner light source to fulfill flashing light effects of microalgae. Bioresour. Technol. 138, 141–147. https://doi.org/10.1016/J.BIORTECH.2013.03.156
Wondraczek, L., Gründler, A., Reupert, A., Wondraczek, K., Schmidt, M.A., Pohnert, G., Nolte, S., 2019. Biomimetic light dilution using side-emitting optical fiber for enhancing the productivity of microalgae reactors. Sci. Reports 2019 91 9, 1–10. https://doi.org/10.1038/s41598-019-45955-w
Rafa, N., Ahmed, S.F., Badruddin, I.A., Mofijur, M., Kamangar, S., 2021. Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front. Energy Res. 9. https://doi.org/10.3389/FENRG.2021.749968/FULL
PBR Images are Courtesy of Pacific Northwest National Laboratory and IGV Biotech under creative commons license.

