Implementation
Proposed Implementation
Overview:
Working on a vaccine for Triple Negative Breast cancer that can work both in a therapeutic and prophylactic manner would’ve never been easy as would be its implementation into the real world as a cost-effective and therapeutic solution to a problem.
However, This year our team tried to anticipate all the upcoming things that might cause a problem and provide a well-studied plan for how our platform can be implemented.
Proposed End Users:
First off we needed to decide & re-evaluate our end users after the many modifications we’ve done to our platform regarding the addition of more safety aspects and increasing the range of users that could benefit from our vaccine.
So The main targets of our vaccine would be:
- People Currently Suffering from TNBC
- People with Breast cancer to prevent its progression to TNBC
- People who can’t tolerate the side effects of regular treatment for breast cancer
- As an adjuvant to regular therapeutic regimens to increase the immune response and success rate However our platform and vaccine could be beneficial to anyone as a prophylactic measure against breast cancer in general and TNBC specifically providing a preformed immunity for them
Proposed implementation:
In the process of turning our idea from a theoretical idea to a practical solution we have worked on many aspects designing a pipeline that we would follow to reach the real world.
In order to achieve that we first needed to work three main milestones which would make the vaccine more producible, safer, and cost effective
Directed Evolution:
First off we worked on the Directed Evolution of our platform creating targeted mutations through multiple iterations to refine and reach the best and most fit (Proteins, Peptides, RNA) on an evolutionary scale. Which could be simply explained by the diagram below.
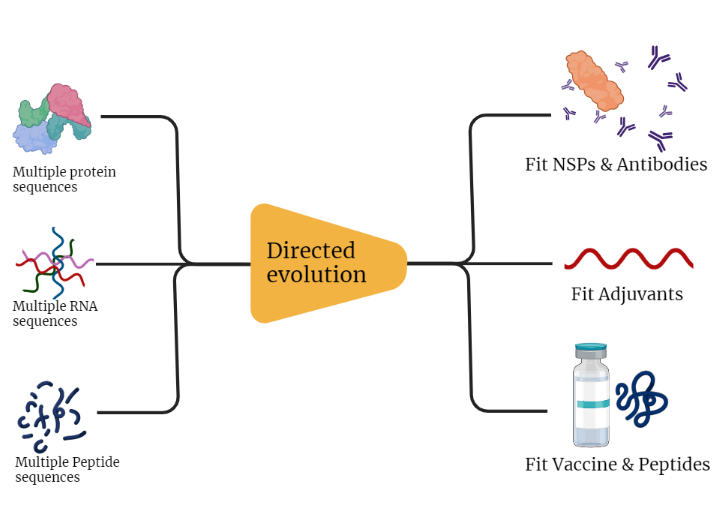
This process would mainly go through many phases:-
- Development Phase: In which we designed different algorithms and classifiers by which we should be able to select the fittest options between multiple optimized sequences
- Validation Phase: In which we would compare our results with other datasets and other work from literature who worked on the same process (In Process)
- Testing Phase: in which we would go through testing our chosen subjects compared to a real world example
- Then we would go through trying to get an In-vitro proof of concept in the lab
- Finally we would integrate what we got with custommune platform
Alphavirus Designing:
Using the enhanced proteins from directed evolution and designing a safer and more stable platform using logic-gating system with addition of more mechanisms that would:-
- Increase the expression and effectiveness of the vaccine
- Provide autoregulatory function to the platform
- Provide methods for the termination of the vaccine
And in order to do that we needed to go through many phases some of which have been done and some are still ongoing.
These phases can be previewed as:-
- Phase I : With the development and optimization of the backbone of our DREP based vaccine which is the NSPs and after modification and enhancement by Directed evolution
- Phase II: an ongoing process now In vitro to try to measure the effectiveness of our vaccine in the lab measuring immune response, cell specificity & expression
- Phase III: with testing of our vaccine in animal models measuring the efficacy of different routes of vaccine administration (IntraTumoral,IM & Intradermal) to choose the best method of administration and moving on to clinical trials I,II,III
Which finally led us to work on…
Optimization of Design & Delivery:
With the use of Viral Like Particles(VLPs) , DNA launched Alpha-viral replicon structure and backbone, and the brilliant designing of the circuit, we made our platform more generalizable, modular and more potent.
To reach our final Design and optimization our vaccine would need to go through multiple Iterations and go through multiple phases to reach its final outcome of optimizing parts of the circuits especially those regarding enhancement of delivery & effectiveness of the vaccine.
This would be transfered into the real world by going through: -Development phase
- Getting a dataset of Potential Peptide candidates
- Those Peptides going through Deep learning tools and algorithms to predict the immunogenicity of them and binding affinity to various types of immune cells
- Repeating the same process till we get the fittest candidates out of the dataset
- Using these candidates in the design of the circuit with VLPs to reach optimum performance and delivery
- Using the candidates as potential vaccine epitopes and chimeric antigens in design
Testing phase
- First we would get to choose our needed metrics and avoid bias to test our
- Choose multiple data from literature to compare and benchmark the fitness and stability of our candidates which have outperformed all the compared data
- Running both In-vitro & In-vivo immunoassays
- Then finally integrate what we got with custommune platform
All of which can be demonstrated in the diagram below:

And the diagram below demonstrates our whole process of implementing our vaccine into a real world application

From There On we knew that our next steps that we would need to work on would be:
- Reaching an In-vitro proof of concept
- Going into animal studies trials
- Then finally through clinical trials I,II,III
And in order to know the best possible way to go in that path we have contacted many researchers and doctors to guide us through the process in order to know how we can do this in the future among those expert minds were:-
Dr.Ahmed Badran, who is a fellow in the Broad Fellows program at the Broad Institute of MIT and Harvard, and is a member of the institute’s Infectious Disease & Microbiome (IDM) and Chemical Biology & Therapeutic Sciences (CBTS) programs. And helped us through his own work and gave us his recommendations regarding the used methodology after presenting the data.

Daniel R. Woldring, who is assistant professor in the chemical engineering and materials science department, as well as guiding us through other aspects regarding protein-protein interaction he helped us form our thoughts about how we can implement this into real life.

In Case and After our vaccine passes all the clinical Trials this would be followed by:-
- Finding an FDA approved Facility to start producing the Vaccine
- Reaching out to stakeholders, angel investors and associations interested in helping with the breast cancer problem
- Getting Funds and the needed Grants to start the manufacturing of material needed to synthesis the vaccine to further complete our trials
Challenges & potential solutions:
In every one of these steps we have been ready and prepared and tried to think of all potential challenges as well as the solutions to them
| Milestone | Potential Challenges |
|---|---|
| Directed Evolution | |
| Alphavirus Designing | |
| Optimization of Design and Delivery |
Potential solutions:
Afterthought we have taken some measures to try and prevent these future challenges before they arise as well as planned some solutions to be taken in the near future such as:
- Reaching out to stakeholders and angel investors to get funds as well as applying for national grants to get the tools we need
- We have contacted multiple doctors and experts in order to guide us through our path
- We have prepared for local courses in the path of training our members with the skills needed to work with more advanced deep learning and directed evolution technologies
- To avoid bias we used multiple and different metrics and datasets as well as randomized ones to stay away from any bias or error
- We have reached out with multiple labs in order to prepare for the upcoming work in the next phases
- Regarding problems with validation we have prepared our self to be able to generate our datasets from different sources to compare our work with others as well as use of different tools to ensure accuracy
- We will plan various animal and clinical models to make sure we pick the most effective route of administration as well as the best and safest design.
Engineering Safety & Risk Assessment and control:
First things first, our project has to be responsible, safe and good for the world. That’s why we had to consider all the safety aspects, possible undesirable outcomes, risks and limitations of our entire project.
How safe is our project? Is our vaccine safe enough? Assessing our current safety procedures and addressing future implementation plans...
Excellence in mathematical modeling enabled us to investigate the canonical correlation between the riboswitch function and performance.
By going in depth through the further applications of riboswitch in the control of gene expression of our vaccine, we propose additional safety procedures that can be equipped by synthetic riboswitches using “Aptazymes”. Incorporating this Off-switch, aptazymes will enable us to shutdown the gene expression of our viral vector in case of unexpectedly emergent risks during clinical application. In a similar manner, we have used TMP as a small molecule inhibitor for the MS2-riboswitch to shut down the circuit in case of undesirable events during in-vivo application.
Why the Viral vector vaccines in particular ?
Working with viral vectors as a potent delivery and shuttle mechanism for introducing our engineered immune-stimulatory antigenic epitopes is considered one of our main concerns. Our viral vector vaccine is engineered to be in-capable of harming the host and with the additional capacity of removing any part of the virus that could induce virulence. As well as being provided with engineered safety switches to stop at any time .As a continuation of our previous efforts last year in engineering and design, we thought about improving the viral-derived non-structural proteins (NSPs) to facilitate the self-replication of our genetic circuit. This was done through lots of iterative rounds using Directed evolution to ensure enhanced functionality and optimum structural stability of these non-structural proteins of viral replicons (nsp1-nsp4).
And this wasn’t our only concerns as we faced additional safety concerns that we needed to tackle such as:-
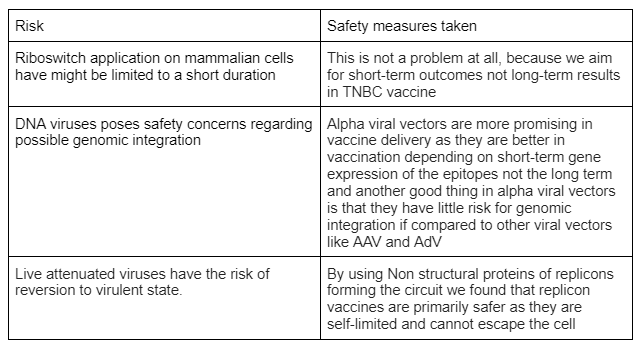
in order to counter any potential risk we add multiple safety measures to our platform which can be both automatic and self regulatory making the platform more system-specific and smart
These design modifications to the circuit could be presented by multiple logic gates that were designed as in the parts BBa_K3743012 & BBa_K3743011 which allows the platform to self regulate its vaccine (Protein) expression either by Positive or negative feedback mechanisms. As in the example below

They work in toggle-switch fashion in a competitive manner where the one with the highest score dominates based on the availability of oncogenic and tumor-suppressive micro-RNAs in the cellular environment. As well as addition of design modifications that would allow for the complete stop of the circuit and inhibition of the vaccine in the presence of TMP that would be injected in case of presence of any unexpected clinical outcome in the setting it was administered in.
Our Vision
Women have played a vital role in the renaissance of old and modern conflicts , and they proved to have a positive impact in solving those problems. The positive change that societies seek depends largely on the contribution of women and the extent to which they can implement changes to society. With the progress and development of communities and cultures. So it was time to give back
Our project is established as a promising solution for Triple Negative breast cancer which is considered as the second cause of cancer-related deaths, preceded by liver cancer. In addition, the most common malignancy among women is breast cancer so our vision is centered on creating a better everyday life for each woman and letting them have the strength to eliminate this malignancy. Not only solve the problem but also treat it safely and dramatically.
Our vaccine could help the women overcome this malignancy without any complications like chemotherapy which achieves our vision to be safe. In addition to being curative, it is also a preventive approach.
We envision our vaccine being used as a personalized therapy to be available in clinics and to help in prevention and combating disease in current survivors of breast cancer from progressing to TNBC and help those who don’t have it early on to be a prophylactic approach to provide with with a better prognosis and hope for possible cure. We expect our vaccine to be a real solution to this problem and to help significantly reduce the huge rates of decades because of this dangerous cancer. And all of this is just the start of our dream.

