Our project represents an attempt for therapeutically managing conditions such as obesity. Although we believe that obese people should not be socially isolated and blamed for their weight and appearance, it is crucial to remember at what cost obese people are living with this condition. The most serious consequences of obesity on health are hypertension, diabetes, myocardial infarction and major cardiovascular events. In particular, diabetes, a consequence of caloric excess, shows a direct association with other comorbidities, such as hypertension which is positively correlated due to vessel damage [1]. Overweight or obesity (BMI ≥25 kg/m2) was found in more than 60% of the adults surveyed in Russia, approaching prevalence levels in the USA, and higher than the prevalence reported in any recent survey in the European Union [2].
Here, we are introducing you to our solution – LEAP2BRAIN.
LEAP2BRAIN is a novel delivery system for the treatment of obesity. We use symbiotic bacteria living in the nose as a delivery platform; it secretes chimeric LEAP2 protein which travels to the hypothalamus region via the trigeminal nerve [3]. LEAP2 is an endogenous antagonist and an inverse agonist of the ghrelin receptor GHS-R1 located in hypothalamus [4][5]. Ghrelin's hallmark functions are its stimulatory effects on food intake, fat deposition and growth hormone release [6]. It is expected that an increase in the ratio of concentrations of LEAP2/Ghrelin in the organism will be beneficial for weight loss, due to the onset of a feeling of satiety and hence a decrease in food intake. The results of a preliminary study on the effect of the LEAP2/ghrelin ratio on mice suggest that LEAP2 may be used as a treatment of Class I and II obesity in humans [7]. However, a separate mouse study showed that systemic administration of LEAP2 had no effect on high-fatdiet intake, whereas centrally injected LEAP2 reduced binge-like high-fat-diet intake. This finding points towards a central mode of action of LEAP2, indicating the need for centrally acting formulations of the compound, for example by intranasal application [8].

Fig.1. Illustration of physiological aspect of LEAP2 and Ghrelin system [4].
Why did we choose LEAP-2? Firstly, we were interested in protein delivery. Secondly, combined with a Myc-tag and LMWP, the fusion protein will have a length of 65 amino acids, which is actually a bit too large for effective penetration into the brain without any additional auxiliary structures, but it’s still small enough for it to be possible to synthesize the gene encoding the protein [9]. Also, the small size of the protein is expected to facilitate secretion into the culture medium. Although we did not have plans on demonstrating the physiological effect of the protein (this is a laborious task that requires a bacterium from the nasal microbiota and carefully calibrated expression regulation), we carried out in silico modelling of the putative structure of the fusion protein to ensure that the Myc-tag and the N-terminus are open, since according to various sources, the first 10-14 amino acids are sufficient for binding to the GHSR1a receptor [10].
Cell penetrating peptides is a fairly rapidly developing topic. Most of these are arginine-rich short N or C-terminal sequences that facilitate the penetration of polar molecules such as proteins and nucleic acids through cell membranes [11]. We plan to use Low molecular weight protamine (LMWP, amino acid sequence VSRRRRRRGGRRRR) attached to the C-terminus of the fusion protein to deliver it from the nose to the brain. A similar experiment has already been carried out, however, the proteins were chemically conjugated to the LMWP, and not secreted by the bacterium [12]. At the same time, Myc-tag is an epitope that allows the identification of ICA proteins by staining, which does not need to be at the end of the protein chain. It will allow us to visualize the results of both secretion into the culture medium and in vivo.
Both for the production of the plasmid (due to the reduced activity of nucleases) and for secretion of the protein (due to the reduced activity of proteases) there’s a need for two different bacteria used as chassis. We selected for these purposes Top10 and BL21 strains respectively. Regarding BL21 strain, normally, when it comes to bacterial secretion of a protein, it is preferable to use the HlyA hemolysin system (Type I), since it provides immediate transport to the extracellular environment. However, in our case, the N-terminus of the protein must remain free, and therefore the LMWP must be attached to the C-terminus, which means that placing an non cleavable signal sequence there is no longer possible. Therefore, we decided to try secretion into the periplasm via the Sec and Tat pathways.
The former ensures the secretion of unfolded protein, while the latter, on the contrary, allows the transport of the folded protein. Both of these pathways have similar cleavable N-terminal secretion signals [13]. This is a somewhat risky decision – there is a great probability that the protein will not be secreted out of the bacteria, so we tried to pick several different leader sequences. Additionally, the influence of LMWP on protein secretion is in itself of considerable interest – there is currently no data on how CPP’s affect the membrane permeability for a protein “from the inside”. We make a control cell line secreting LEAP2 + Myc-tag without LMWP. Other types of secretion systems are found mostly in pathogenic microorganisms. Some of them have complex secretion signals, in some cases, the secretion signals are not cleaved, like in type I, some systems are not well understood, and an attempt of their use is in itself enough reason for a separate project.
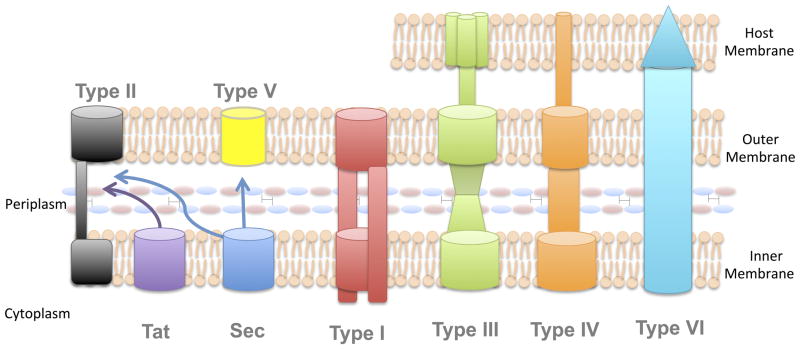
Fig. 2. Secretion systems in Gram-negative bacteria [13].
So, down below in the picture, you can see our plasmid construct design. There are 10 of them in total, but the 2 codings of the YebF protein seem to be off to the side. They don’t encode the Leap-2, why do we need them? YebF is a small (10,8 kDa) protein, which is known to be secreted into the culture medium by E. coli K12. Hence, we will be able to evaluate the influence of the LMWP on protein secretion. You may say that the experiment is not clear, because the Myc-tag is presented in the control construct. But it has been shown for the YebF that it can carry passenger proteins fused to its C-terminus out of the bacteria [14].

Fig. 3. Plasmid construct design.
The rest 8 constructs can be also divided into 2 groups: those with no LMWP (controls) and those carrying it. This is due to our desire to estimate whether the LMWP can increase the membrane permeability of the fusion proteins in case of bacterial secretion. Speaking of the Sec and Tat leaders choice we have PelB, because it is a well-known secretion tag in molecular microbiology and in the lab where we are working. It is said, that even without the LMWP it manages to provide a little amount of the extracellularly secreted protein TorA was the only Tat secretion leader we did find in the Registry and the most well-documented one [15]. We also tried to modify PelB and TorA leaders (PelB* and TorA* on the Fig.3 respectively) using the TatP and Phobius software tools for secretion leaders prediction [16][17].
The expression plasmid (pSG4K5) and promoter (J23119) choice is motivated by the following considerations: we can’t use an inducible promoter in anyone’s nose, thus we need a constitutive one. The J23119 is rather strong, but pSG4K5 compensates this with a low-copy origin so the bacteria probably won’t die from overproducing the protein.
Building up a regulatory pathway is extremely important for the living therapeutics design. But the choice of meal-connected signals in the nasal cavity is extremely poor. We consider a great result of our brainstorming, that we have discovered an alternative: why do we need an outer signal, if we can just help people stick to their diets? We are going to make our bacteria secrete Leap-2 periodically, increasing the appetite when it is dinner or breakfast, and decreasing it after the meal is taken to prevent uncontrolled snacking. As long as we know it is the first living therapeutics project exploiting the genetic oscillator for the regulation. We believe there would be more of them, because circadian rhythms play a crucial role in our health, so why not use them?

Fig. 4. Expected production and secretion pattern of chimeric protein.
The article, on which we based our idea of making a fusion protein with the cell-penetrating peptide, LWMP, [12] states that the mechanism by which their fusion protein travels to the hypothalamus via the olfactory and trigeminal nerves is unclear. It is speculated that endocytosis and macropinocytosis play an important role, however, the intra-tissue penetration mechanisms are “largely unknown” and most likely occur via an extracellular mechanism. Therefore uncovering the underlying mechanism behind this type of delivery is a point of interest to us, and an interesting area of research in itself. To account for both potential mechanisms we decided to use two simple in vitro models. You can read more about it on the Implementation page.
References
[1] De Lorenzo, Antonino et al. “Why primary obesity is a disease?.” Journal of translational medicine vol. 17,1 169. 22 May. 2019, doi:10.1186/s12967-019-1919-y
[2] Kontsevaya A, Boytsov S et al. “Overweight and Obesity in the Russian Population: Prevalence in Adults and Association with Socioeconomic Parameters and Cardiovascular Risk Factors”. Obes Facts. 2019;12(1):103-114. doi: 10.1159/000493885.
[3] Jansson, B. (2004). Models for the Transfer of Drugs from the Nasal Cavity to the Central Nervous System (PhD dissertation, Acta Universitatis Upsaliensis). Retrieved from http:// urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-3905
[4] Ge X, Yang H, Bednarek MA, et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018;27(2):461-469.e6. doi:10.1016/j.cmet.2017.10.016
[5] M’Kadmi C, Cabral A, Barrile F, et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J Med Chem. 2019;62(2):965-973. doi:10.1021/acs.jmedchem.8b01644
[6] Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16(6):619-624. doi:10.1097/MCO.0b013e328365b9be.
[7] Gupta, Deepali et al. “"A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes".” Molecular metabolism vol. 46 (2021): 101128. doi:10.1016/j.molmet.2020.101128
[8] Schalla, M.A., Stengel, A. LEAP2: A novel regulator of food intake and body weight?. Nat Rev Gastroenterol Hepatol 16, 711–712 (2019). https://doi.org/10.1038/s41575-019-0224-9 [9] Darshana S , June S. “Nasal Delivery of Proteins and Peptides”. Glob J Pharmaceu Sci. 2017; 1(4) : 555569. DOI: 10.19080/GJPPS.2017.01.555569
[10] Céline M’Kadmi, Mario Perello, and Jean-Alain Fehrentz et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. Journal of Medicinal Chemistry 2019 62 (2), 965-973 DOI: 10.1021/ acs.jmedchem.8b01644
[11] Kurrikoff, Kaido, and Ülo Langel. “Recent CPP-based applications in medicine.” Expert opinion on drug delivery vol. 16,11 (2019): 1183-1191. doi:10.1080/17425247.2019.1665021
[12] Lin, Tingting et al. “Nose-to-brain delivery of macromolecules mediated by cell-penetrating peptides.” Acta pharmaceutica Sinica. B vol. 6,4 (2016): 352-8. doi:10.1016/j.apsb.2016.04.001
[13] Green, E. R., and Mecsas, J. (2016). Bacterial secretion systems?: an overview. Microbiol. Spectr. 4:10.1128/microbiolsec.VMBF–0012–2015. doi: 10.1128/microbiolspec.VMBF-0012-2015
[14] Zhang, G., Brokx, S. & Weiner, J. Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli. Nat Biotechnol 24, 100–104 (2006). https://doi.org/ 10.1038/nbt1174
[15] Hideki Fukuda et al. Efficient secretory overexpression of Bacillus subtilis pectate lyase in Escherichia coli and single-step purification. Biochemical Engineering Journal. 2002. https:// doi.org/10.1016/S1369-703X(02)00075-X
[16] Bendtsen, J.D., Nielsen, H., Widdick, D. et al. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6, 167 (2005). https://doi.org/10.1186/1471-2105-6-167
[17] Lukas Käll, Anders Krogh, Erik L.L Sonnhammer. A Combined Transmembrane Topology and Signal Peptide Prediction Method. Journal of molecular biology. 2004. https://doi.org/10.1016/ j.jmb.2004.03.016
SIBIRIA

